Collecting & Submitting Specimens At Time of Birth for Zika Virus Testing
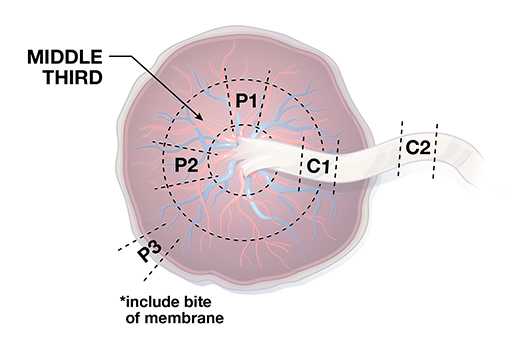
Placental Specimen
General Information
Laboratory testing for congenital Zika virus infection is recommended for infants born to mothers with laboratory evidence of Zika virus infection during pregnancy, and for infants who have abnormal clinical findings suggestive of congenital Zika virus syndrome and a maternal epidemiologic link suggesting possible transmission, regardless of maternal Zika virus test results.
For infants born to mothers with risk factors for maternal Zika virus infection (travel to or residence in an area with risk of Zika virus transmission or sex with a partner with travel to or residence in such an area) for whom maternal testing was not performed before delivery, assessment of the infant, including comprehensive physical exam and careful measurement of head circumference, neurologic assessment as well as newborn hearing screen should be performed. In addition, based on the level of possible maternal Zika virus exposure (e.g., duration and type of exposure, use of prevention measures, intensity of Zika virus transmission at the location of travel), the provider should consider whether further evaluation of the newborn for possible congenital Zika virus infection is warranted, in which case a head ultrasound and ophthalmologic assessment should be considered. Based on results of the evaluation, testing of the infant for Zika virus infection could be considered.
Testing of placental tissue specimens by Zika virus reverse-transcription polymerase chain reaction (RT-PCR) is conducted at CDC’s Infectious Diseases Pathology Branch (IDPB). Zika virus RT-PCR on placental tissues from women with possible Zika virus exposure can be considered for diagnostic purposes for symptomatic pregnant women and women with infants with possible Zika virus–associated birth defects without a definitive diagnosis of laboratory-confirmed Zika virus infection during pregnancy. For asymptomatic pregnant women who have recent possible Zika virus exposure but without ongoing possible exposure, similar to the updated recommendations for testing of non-tissue clinical specimens (e.g., serum and urine), testing of placental tissues is not routinely recommended.
IMPORTANT: Pre-approval is required prior to submission of any tissue specimens. For pre-approval please contact pathology@cdc.gov.

Collection and Submission of Specimens for Zika Virus Testing at Time of Birth
Healthcare Providers
- Please make sure that your state, territorial, tribal, or local health department has been notified and has received pre- approval from CDC for submission and shipment of tissue specimens before they are collected and sent.
- Institutions with surgical pathology available: Please consult surgical pathology regarding appropriate collection and processing of tissue specimens for Zika virus testing.
- Institutions without surgical pathology available: Please see table below for general guide on collection of tissue specimens for Zika virus testing.
- Specimens should ONLY be sent to CDC directly from health departments. CDC’s Zika Pregnancy Hotline (ZikaMCH@cdc.gov) is available 24/7 to health care providers and health departments for consultation regarding management of pregnant women and infants with possible Zika virus. This hotline can also assist with questions regarding specimen submission. Healthcare providers and state and local health officials email ZikaMCH@cdc.gov.
Health Departments
- When submitting specimens, please submit CDC Form 50.34 with all specimens.
- Select Test Order Code CDC-10365 “Pathologic Evaluation of Tissues for Possible Infectious Etiologies.”
- Select “Zika Virus” as the suspect agent from the drop down menu.
- The remaining items must be completed electronically and then printed.
- Pre-approval is required prior to submission of all tissue specimens (i.e. placenta, umbilical cord). Please contact pathology@cdc.gov to obtain pre-approval. Additional clinical and epidemiologic information may be requested, see https://www.cdc.gov/zika/laboratories/test-specimens-tissues.html for minimum required information for pre-approval. If you have additional questions for Infectious Diseases Pathology Branch please call 404-639-3133.
- If you have additional questions for the Arboviral Diseases Branch, please call 970-221-6400.
Reporting of Results
- Test results will be reported to the state health department and the submitting healthcare provider. Results will not be directly released to patients.
- Turnaround time will depend on testing volume and established reporting systems.
| Specimen Type | General Instructions | Notes | Storage | Shipping |
|---|---|---|---|---|
| Infant serum |
|
|
|
|
| Placenta and fetal membranes |
|
|
|
|
| Umbilical cord |
|
|
|
|
| Infant urine |
|
|
|
|
- Page last reviewed: October 3, 2017
- Page last updated: October 3, 2017
- Content source:





 ShareCompartir
ShareCompartir