Acid Detection Test
Principle
Patterns of acid production from the carbohydrates - glucose, maltose, lactose, sucrose, (and fructose) - are used to identify Neisseria and related species. In contrast to most bacteria which produce acid by a fermentative pathway, Neisseria spp. produce acid by an oxidative pathway. This is an important distinction because more acid is produced by fermentation than by oxidation. Some Neisseria species, e.g., Neisseria cinerea, produce acid from carbohydrates but rapidly overoxidize the acid to carbon dioxide, with the result that acid does not accumulate in the reaction tube although the carbohydrate has been used; N. cinerea is considered to be glucose-negative. In addition to producing less acid from carbohydrates, many Neisseria spp. produce ammonia from peptone which may neutralize the acid produced from carbohydrates. "Acid detection' is probably the most accurate term for describing the results obtained in tests designed to detect acid production from carbohydrates. The terms 'carbohydrate utilization' and 'fermentation' should not be used to describe acid production by Neisseria spp.
The traditional test for determining acid production by Neisseria and related species has been the Cystine Trypticase Agar (CTA) test in which a base medium was supplemented with a 1% (final concentration) filter-sterilized carbohydrate solution adjusted to a final pH of 7.3. Phenol red indicator is added to detect acid; this indicator changes from red (alkaline) to yellow (acid). CTA media, contained in screw-capped tubes, are inoculated with heavy suspensions of a 18 h-24 h pure subculture from chocolate, or equivalent, medium. The tubes are then incubated, without supplemental carbon dioxide, at 35C-36.5C for 24 h. Often, tests must be incubated for 24h-48 h. before the reaction patterns can be determined.
CTA-carbohydrate tests are no longer recommended for detecting acid produced by Neisseria species. CTA-carbohydrate media are designed to detect acid production from fermentative organisms which, as noted above, produce more acid from carbohydrates than do the oxidative Neisseria spp. It is also intended that organisms produce acid as they grow in CTA-carbohydrate media; strains of Neisseria spp., including N. gonorrhoeae, do not grow well in these media. Because the proportion of peptone to carbohydrate is high in CTA medium and because Neisseria spp. have produced ammonia from peptone and neutralized the acid produced from carbohydrates, it may be difficult to obtain accurate results . Thus, alternative media have been developed that either contain a lower ratio of peptone to carbohydrate or that use carbohydrate solutions which are inoculated with heavy suspensions and depend on acid being produced by preformed enzymes present in the suspension.
Rapid Acid Detection Tests
Several rapid tests have been developed to detect acid production from carbohydrates by Neisseria spp. These tests depend on preformed enzymes present in heavy suspensions of the microorganism to produce detectable acid from carbohydrates. Phenol red is used as the pH indicator in most rapid acid detection tests for Neisseria spp. Organisms which produce acid from carbohydrates will produce sufficient acid to exceed the buffering capacity of the substrate medium and cause a color change from red (alkaline) to yellow (acid).
An example of a rapid acid detection test is QuadFERM+, shown in Figure 1. QuadFERM+ is a plastic strip composed of a cupule containing a carbohydrate-free medium control and cupules containing carbohydrate substrates to detect acid production from glucose, maltose, lactose, and sucrose. QuadFERM+ also includes other differential tests--deoxyribonuclease, DNase, and beta-lactamase tests--for Neisseria and related species. Organisms which produce acid from carbohydrates will produce sufficient acid to exceed the buffering capacity of the substrate medium and cause a color change from red (alkaline) to yellow (acid), e.g., Figure 1.
Figure 1. Example of a rapid acid detection test for Neisseria and related species.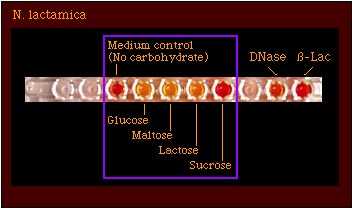
Specimen Requirements
Optimum specimen: Pure culture (18h-24 h.) of gram-negative, oxidase- positive, catalase-positive diplococci grown on nonselective (chocoate or equivalent) medium isolated either from a selective (Modified Thayer-Martin; MTM) medium or a nonselective (chocolate or equivalent) medium.
Unacceptable specimens:
- Contaminated cultures grown on nonselective medium
- Apparently pure cultures grown on selective medium
- Pure cultures older than 18h-24 h. grown on nonselective medium
Factors that compromise test results:
- Use of contaminated cultures to inoculate test.
- Incubation of tests in an incubator with carbon dioxide-supplemented atmosphere. Carbon dioxide may diffuse into the medium and form carbonic acid which may cause a false-positive acid reaction.
Stability of inoculum:
- Acid production in this test is dependent on preformed enzymes. Media should be inoculated within 30 min. of removing culture from incubator; prolonged exposure at room temperature may result in diminished enzyme activity.
Medium/Reagents
Acid production tests for Neisseria and related species routinely test for acid production from glucose, maltose, sucrose, and lactose. Tests to detect acid production from fructose are performed primarily for research purposes or by reference laboratories.
QuadFERM+: Commercial acid detection test (for glucose, maltose, sucrose, and lactose) that includes a DNase and beta-lactamase test.
Store in accordance with manufacturer's directions.
Quality Control/Test Procedure
QC strains:- N. gonorrhoeae, strain ATCC 31426
- N. lactamica, strain ATCC 23971
- M. catarrhalis, strain ATCC 25240
- N. mucosa, strain ATCC 19695
QC strains are stored at -70C in a solution of trypic soy broth containing 20% glycerol. Reactions of QC strains should be confirmed at the time the frozen stocks are prepared. QC strains may be stored at -70C for 2 years.
Procedure:
- Thaw vials of QC strains stored at -70C. Clinical isolates may be subcultured from selective medium or purified subcultures.
- Streak-inoculate strains onto plates of chocolate agar (or equivalent medium) for isolation. Incubate plates at 35C-36.5C in a carbon dioxide-enriched (5%) atmosphere for 18 to 24 h. Confirm that cultures are pure.
Perform tests as described in the manufacturer's directions. Phenol red is used as the pH indicator in most products. An orange-red color is negative; a yellow color is positive.
Positive reaction is recorded when the color in the test medium is more orange/yellow than the color in the control medium.
Negative reaction is recorded when the color in the test medium is the same, or a darker red, than the color in the control medium.
- Read and record results. The results for the QC strains are shown in Table 1.
- To identify the test strains, compare the reaction patterns of the test strain with those given in the product insert.
Note: Because several Neisseria species may give the same acid detection patterns (Table 2), it may be necessary to perform additional tests to confirm the identity of the test strain.
Table 1. Acid production patterns of quality control reference strains of N. gonorrhoeae, N. lactamica, N. mucosa, and M. catarrhalis
| QC Strain | Acid Reaction Pattern |
|---|---|
N. gonorrhoeae, ATCC 31426 Acid only from glucose |  |
N. lactamica, ATCC 23971 Acid from glucose, maltose, and lactose |  |
N. mucosa, ATCC 19695 Acid from glucose, maltose, and sucrose |  |
M. catarrhalis, ATCC 25240 No acid produced |  |
Quality Control Schedule:
- Each new lot of quadFERM+ is tested upon receipt from the manufacturer.
- Note: If more than one lot number is received at the same time both lots must be tested.
- Note: If two of the same lot numbers are received at different times, each shipment must be tested.
Table 2. Acid detection patterns of Neisseria and related species.
| Species that produce acid from: | Illustration |
|---|---|
Glucose only N. gonorrhoeae |  |
Glucose and Maltose N. meningitidis |  |
Glucose, Maltose, and Lactose |  |
Glucose, Maltose, and Sucrose N. mucosa |  |
No Carbohydrate |  |
This section contains some suggestions for why accurate test results may not be obtained in acid detection tests together with some explanations and solutions for these problems.
All tests are acid. Make sure tests are incubated in an atmosphere without supplemental carbon dioxide atmosphere. Carbon dioxide may dissolve in the media, form carbonic acid, and cause an acid reaction in the media.
Weak test results. Tests should be warmed to room temperature before use; tests rely on preformed enzymes. Inoculation of cold test strips may reduce the activity of the enzymes.
Incorrect test results. USE PURE CULTURES ONLY. Mixed cultures may include organisms that produce acid from carbohydrates different from those of the test neisserial strain.
Incorrect test results. Prepare inocula as described in manufacturer's directions. Preparation of suspensions in saline containing preservatives, buffers, or bacteriostatic agents should NOT be used in preparing bacterial suspensions; these may denature the preformed enzymes that react with the substrates or neutralize acid produced by organisms. DO NOT make bacterial suspensions in water; enzymes may be denatured.
Weak or incorrect results. INOCULATE BACTERIAL SUSPENSIONS IMMEDIATELY (within 15 min. after preparation from pure cultures that have been held at room temperature no longer than 30 min.) AFTER PREPARATION: Enzymes may denature if suspensions are not used immediately. If many strains are to be tested, inoculate each test strip immediately after preparation of the suspension rather than preparing all suspensions and then inoculating tests; this inoculation strategy will also minimize the possibility of mislabeling a test strip.
Acid production tests cannot be used to identify all Neisseria and related species strains isolated from either selective or nonselective media. Problems have been associated with the identification of these species in acid detection tests for two reasons.
Several species exhibit the same acid production patterns
Some strains may exhibit aberrant acid patterns; these species include N. meningitidis (maltose-negative variants), N. cinerea (overoxidizes acid produced from glucose but may exhibit a weak acid reaction due to the formation of carbonic acid), and N. lactamica (lactose-negative variant [very rare]).
Thus, with the exception of N. lactamica strains that produce acid from glucose, maltose, and lactose, additional tests must be performed before a CONFIRMED/DEFINITIVE identification may be reported.
Examples of additional tests that may be used to differentiate between species exhibiting similar acid production patterns are shown in Tables 3, 4, 5, and 6.
Additional Tests
Table 3. Supplemental tests which permit differentiation among gram-negative diplococci that produce acid only from glucose.
| Species that produce acid only from glucose | Gram Stain | Enzyme substrate test | Nitrate Reduction | Colistin Resistance |
|---|---|---|---|---|
| N. gonorrhoeae | GND | Hydroxy- prolylaminopeptidase +ve | - | R |
| K. denitrificans | GND | Hydroxy- prolylaminopeptidase +ve | + | R |
| Maltose -ve N. meningitidis | GND | Gamma-glutamyl- aminopeptidase +ve | - | R |
| "N. kochii" | GND | Hydroxy- prolylaminopeptidase +ve | - | R |
| (N. cinerea) | GND | Hydroxy- prolylaminopeptidase +ve | - | (R) |
| (N. elongata) | GNR | Hydroxy- prolylaminopeptidase +ve | - | (S) |
Abbreviations: GND, Gram-negative diplococcus; GNR, Gram-negative rod; +, most strains positive; -, most strains negative; R, strains grow well on selective medium for N. gonorrhoeae and/or show no inhibition around a colistin disk (10 micrograms); (R), most strains susceptible, some strains known to be resistant; S, most strains sensitive, no strains known to be resistant.
Although enzyme substrate tests are intended to be used only for the identification of Neisseria spp. isolated on selective media for N. gonorrhoeae, these tests do provide additional information that may aid in accurately identifying an isolate. However, these tests should not be used as the primary identification test for strains isolated on nonselective media.
Table 4. Supplemental tests which permit differentiation among gram-negative diplococci that produce acid from glucose and maltose.
| Species that produce acid from glucose and maltose | Enzyme substrate test | Polysaccharide from Sucrose | Colistin Resistance |
|---|---|---|---|
| N. meningitidis | Gamma-glutamyl- aminopeptidase +ve | - | R |
| N. polysaccharea | Hydroxy- prolylamino- peptidase +ve | + | (R) |
| N. subflava biovar subflava biovar flava | Hydroxy- prolylamino- peptidase +ve | - | S |
| (Lactose-negative N. lactamica) | Beta-galactosidase +ve | - | R |
Abbreviations: GND, Gram-negative diplococcus; GNR, Gram-negative rod; +, most strains positive; -, most strains negative; R, strains grow well on selective medium for N. gonorrhoeae and/or show no inhibition around a colistin disk (10 micrograms); (R), most strains susceptible, some strains known to be resistant; S, most strains sensitive, no strains known to be resistant.
Although enzyme substrate tests are intended to be used only for the identification of Neisseria spp. isolated on selective media for N. gonorrhoeae, these tests do provide additional information that may aid in accurately identifying an isolate. However, these tests should not be used as the primary identification test for strains isolated on nonselective media.
Table 5. Supplemental tests which permit differentiation among gram-negative diplococci that produce no detectable acid from carbohydrates.
| Species that produce no detectable acid | Gram Stain | Enzyme substrate test | DNase | Superoxol | Poly- saccharide from sucrose | Colistin susceptibility |
|---|---|---|---|---|---|---|
| (Glucose-negative N. gonorrhoeae) | GND | Hydroxy- prolylamino- peptidase +ve | - | Strong 4+ | - | R |
| M. catarrhalis | GND | No reaction | + | Strain variable (1+ to 4+) | - | (R) |
| N. cinerea | GND | Hydroxy- prolylamino- peptidase +ve | - | Weak (2+) | - | (R) |
| N. elongata | GNR | Hydroxy- prolylamino- peptidase +ve | - | - | - | S |
| N. flavescens | GND | Hydroxy- prolylamino- peptidase +ve | - | Weak (2+) | + | S |
Abbreviations: GND, Gram-negative diplococcus; GNR, Gram-negative rod; +, most strains positive; -, most strains negative; R, strains grow well on selective medium for N. gonorrhoeae and/or show no inhibition around a colistin disk (10 micrograms); (R), most strains susceptible, some strains known to be resistant; S, most strains sensitive, no strains known to be resistant.
Although enzyme substrate tests are intended to be used only for the identification of Neisseria spp. isolated on selective media for N. gonorrhoeae, these tests do provide additional information that may aid in accurately identifying an isolate. However, these tests should not be used as the primary identification test for strains isolated on nonselective media.
Table 6. Supplemental tests which permit differentiation among gram-negative diplococci that produce acid from glucose, maltose, and sucrose.
| Species that produce acid from glucose, maltose, and sucrose | Nitrate Reduction | Colony Morphology | Pigmentation |
|---|---|---|---|
| N. mucosa | + | Butyrous, smooth | - |
| N. subflava biovar perflava | - | Butyrous, smooth | Yellow |
| N. sicca | - | May become wrinkled, adherent | - |
Abbreviations: +, most strains positive; -, most strains negative.
Reporting
Results obtained in acid detection tests permit the confirmed/definitive identification of some Neisseria and related species and the presumptive identification of others. With the exception of Kingella denitrificans, the identifications made on the basis of this test assume, that isolates are gram-negative, oxidase-positive diplococci.
Confirmed/definitive identification: of Neisseria lactamica may be made directly on the basis of the results of acid detection tests irrespective of the medium on which the strains were isolated. N. lactamica is the only Neisseria spp. from man or animal that produces acid from lactose.
Other Neisseria and related species may give similar reactions in acid detection tests. These species include N. gonorrhoeae. Thus, results of acid detection tests must be reported as Presumptive, unless supplemental tests (see Tables 3, 4, 5, and 6 above) are performed to identify the isolate accurately.
References
Bovre K. Family VIII. Neisseriaceae Prevot. In Krieg NR, ed. Manual of Systematic Bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore; 1984:288-309.
Knapp JS. Historical perspectives and identification of Neisseria and related species. Clin Microbiol Rev 1988;1:415-431.
Knapp JS, Rice RJ. Neisseria and Branhamella. In. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, ed. Manual of Clinical Microbiology. 6th ed. American Society for Microbiology, Washington D. C.; 1995:321-340.
Vedros NA. Genus I. Neisseria Trevisan 1885, 105AL. In Krieg NR, ed. Bergey's Manual of Systematic Bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore; 1984:290-296.
- Page last reviewed: December 10, 2013
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir