Moraxella catarrhalis
Introduction
Moraxella catarrhalis is classified with the genera Neisseria, Moraxella, Kingella, and Acinetobacter in the family Neisseriaceae. The taxonomic position of M. catarrhalis is currently being debated; it has been proposed that M. catarrhalis be assigned to the genus Moraxella (M. catarrhalis) in the family Moraxellaceae, or to its own genus, Branhamella, in the family Branhamaceae. In the early 1900s, descriptions of "N. catarrhalis" in the normal oropharyngeal flora were probably incorrect. Before 1962, when N. cinerea was described, strains of this species were misidentified as N. catarrhalis. In spite of questions regarding the taxonomic status of this species, in the clinical laboratory, isolates of M. catarrhalis must be distinguished from Neisseria spp.
Colonies of M. catarrhalis may have a rough surface and be friable in consistency, pinkish-brown in color, and opaque. Whereas Neisseria spp. have an optimal growth temperature of 35C-37C, M. catarrhalis strains tolerate lower temperatures and grow well at 28C. M. catarrhalis is not frequently isolated from the oropharynx of healthy adults, but may be carried more frequently in children and older adults.
Although M. catarrhalis has been recognized as a cause of human infections since the early 1900s, the variety of infections caused by this species has only been documented clearly in the past 10 to 15 years. M. catarrhalis causes acute, localized infections such as otitis media, sinusitis, and bronchopneumonia as well as life-threatening, systemic diseases including endocarditis and meningitis. M. catarrhalis also causes a large proportion of cases of lower respiratory tract infections in elderly patients with chronic obstructive pulmonary diseases and chronic bronchitis and frank pneumonia. The mechanisms by which M. catarrhalis causes infections are not understood.
Characteristics of M. catarrhalis
| Characteristic | Illustration |
|---|---|
| Gram stain Cell Morphology | Gram-negative diplococcus |
| Colony Morphology | 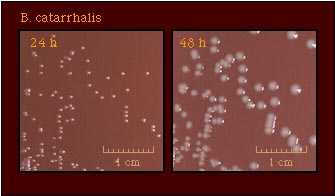 |
| Pigmentation | 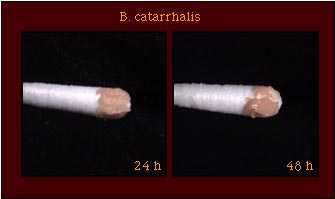 |
| Oxidase Test | 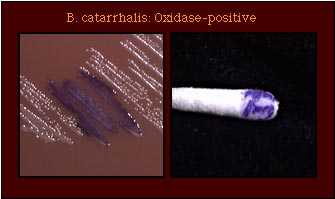 |
| Acid Production |  |
| Enzyme Substrate Test | No reaction |
| Nitrate Reduction Test | Nitrate +ve |
| Polysaccharide from Sucrose |  Polysaccharide -ve |
| Production of Deoxyribonuclease (DNase) |  DNase-positive +ve |
| Superoxol Test (Reaction with 30% hydrogen peroxide) |  Strain variable; reactions vary from weak (1+) to very strong (4+) |
Catalase Test When performed on a clear base medium without hemoglobin, the catalase reaction of M. catarrhalis may occur vigorously, but very rapidly and then become "negative;" persistent bubbles may not be observed. Thus, the reaction should be watched to observe a positive catalase reaction. |  Catalase-positive |
| Colistin Resistance |  Most strains are colistin-sensitive; some strains are colistin-resistant. |
Species which may be misidentified as M. catarrhalis in acid detection tests
Based on acid production patterns, M. catarrhalis may be misidentified as N. cinerea, N. flavescens, or as a glucose-negative strain of N. gonorrhoeae. Supplemental tests that may aid in differentiating between species that produce no detectable acid from carbohydrates are shown in Table 2.
Table 2. Supplemental tests which permit differentiation among gram-negative diplococci that produce no detectable acid from carbohydrates.
| Species that produce no detectable acid | Gram Stain | Enzyme Substrate | DNase | Superoxol | Polysaccharide from sucrose | Nitrate reduction | Pigment | Colistin susceptibility |
|---|---|---|---|---|---|---|---|---|
| M. catarrhalis | GND | No reaction | + | Strain variable (1+ to 4+) | - | + | - | (R) |
| (Glucose-negative N. gonorrhoeae) | GND | Hydroxy prolylamino- peptidase +ve | - | Strong 4+ " Explosive" | - | - | - | R |
N. cinerea | GND | Hydroxy prolylamino- peptidase +ve | - | Weak (2+) | - | - | - | (R) |
N. elongata | GNR | Hydroxy prolylamino- peptidase +ve | - | - | - | - | - | S |
N. flavescens | GND | Hydroxy prolylamino- peptidase +ve | - | Weak (2+) reaction | + | - | + | S |
Abbreviations: GND, Gram-negative diplococcus; GNR, Gram-negative rod; +, most strains positive; -, most strains negative; R, strains grow well on selective medium for N. gonorrhoeae and/or show no inhibition around a colistin disk (10 micrograms); (R), most strains susceptible, some strains not known to be resistant; S, most strains sensitive, none known to be resistant.
Although enzyme substrate tests are intended to be used only for the identification of Neisseria spp. isolated on selective media for N. gonorrhoeae, these tests do provide additional information that may aid in accurately identifying an isolate. However, these tests should not be used as the primary identification test for strains isolated on nonselective media. M. catarrhalis is unique among gram-negative diplococci; strains of M. catarrhalis produce no reaction in enzyme substrate tests.
References
Bovre K. Family VIII. Neisseriaceae Prevot. In Krieg NR, ed. Manual of Systematic Bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore; 1984:288-309.
Knapp JS. Historical perspectives and identification of Neisseria and related species. Clin Microbiol Rev 1988;1:415-431.
Knapp JS, Rice RJ. Neisseria and Branhamella. In. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, ed. Manual of Clinical Microbiology. 6th ed. American Society for Microbiology, Washington D. C.; 1995:321-340.
Vedros NA. Genus I. Neisseria Trevisan 1885, 105AL. In Krieg NR, ed. Bergey's Manual of Systematic Bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore; 1984:290-296.
- Page last reviewed: March 31, 2017
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir