Enzyme Substrate Test
The production of three enzymes - a glycosidase (beta-galactosidase) and two aminopeptidases (gamma-glutamylaminopeptidase and hydroxyprolylaminopeptidase) - has been used to differentiate between Neisseria and related species isolated on selective medium for N. gonorrhoeae.
Although this test is easy to perform, nongonococcal isolates including commensal Neisseria spp. and K. denitrificans, which may grow on selective media, may be misidentified as N. gonorrhoeae if additional tests are not performed. Enzyme substrate tests should not be used as the only test for identifying N. gonorrhoeae, particularly if such an identification may have medicolegal implications.
Principle
Gonochek II is a tube test that is designed to differentiate between Neisseria lactamica, N. meningitidis, N. gonorrhoeae and Moraxella catarrhalis. The enzymes produced by these species are detected in a single test by the production of colored endproducts from colorless substrates. Beta-galactosidase, produced by N. lactamica strains, hydrolyzes 5-bromo-4-chloro-3-indolyl-beta-D-galactoside (BCIG) to produce a blue-colored enproduct. N. meningitidis produces gamma-glutamylaminopeptidase which hydrolyzes gamma-glutamyl-p-nitroanilide (GPNA) releasing a yellow p-nitroaniline endproduct. Hydroxyprolylaminopeptidase, produced by N. gonorrhoeae strains, hydrolyzes a beta-naphthylamino acid derivative (prolyl-4-methoxynaphthylamide [PMNA]) and releases a free beta-naphthylamine derivative which then complexes with a diazonium salt to produce a red-pink color. M. catarrhalis has none of these enzymes; therefore, strains of M. catarrhalis produce no distinct color change in this test.
Specimen Requirements
Optimum specimen: Well-isolated colonies of a 24-48 h. pure culture, on chocolate (or equivalent) medium of gram-negative, oxidase-positive diplococci that have been isolated on a selective medium (Modified Thayer-Martin or equivalent medium) for N. gonorrhoeae.
Unacceptable specimen:
- 24-48 h. cultures grown on gonococcal selective media without demonstration of purity by passage on a nonselective medium;
- Cultures greater than 48 h. grown on nonselective medium even if isolated on selective medium;
- Cultures of organisms that are not gram-negative, oxidase-positive diplococci.
Compromising factors affecting test results: The test must be inoculated with a pure culture of the isolate.
- Contamination with other strains may produce aberrant results if the contaminating strain(s) produce any of the three enzymes being detected in this test.
- "Dilution" of inoculum with a strain that does not produce an enzyme detected in this test may result in failure to detect enzyme produced by a neisserial isolate.
- Activity of enzymes may be diminished/absent in cultures which have been incubated longer than 48 h., particularly if cells autolyze. Use only well isolated colonies of a 24-48 h. culture to inoculate test.
Stability of specimen:
- Test should be performed within 30 min. of removal of culture from incubator. Enzyme activity may diminish with time upon removal of culture from incubator to room temperature.
Medium/Reagents
Gonochek-II: Commercial enzyme substrate test
Storage conditions: Store in the dark at 0C to 8C (Refrigerated).
Shelf life: Do not use after the expiration date or if the substrate is visibly wet.
Quality Control/Test Procedure
QC strains:
- Neisseria gonorrhoeae, strain ATCC 19424
- Neisseria meningitidis, strain ATCC 13077
- Neisseria lactamica, strain ATCC 23970
- Moraxella catarrhalis, strain ATCC 25238
QC strains are stored at -70C in a solution of tryptic soy broth containing 20% glycerol. Reactions of control strains should be confirmed at the time frozen stocks are prepared. QC strains may be stored at -70C for 2 years.
Procedure:
Clinical isolates and QC strains are tested in the same manner according to the manufacturer's directions. QC strains should be subcultured at least once after the initial culture from the frozen specimen before the test is performed.
- Streak-inoculate strains onto plates of chocolate agar (or equivalent medium) for isolation. Incubate plates at 35C to 36.5C in a carbon dioxide-enriched (5%) atmosphere for 18-24 h. Confirm that cultures are pure.
- Allow tests to warm to room temperature before use.
- Remove, as a pair, red and translucent, stoppers from the Gonochek-II tube.
- Use a preservative-free transfer pipette, dispense 4 drops of PBS into the tube.
- Using a wooden applicator stick, harvest the equivalent of 5-10 fresh, medium-to-large size colonies of similar morphology and emulsify well in the PBS in the Gonochek-II tube. Best results will be obtained with growth from well-isolated colonies that have not lyzed.
- Recap the tube with the pair of stoppers.
- Incubate the capped tube in a 35C to 36.5C incubator (or heat block) for 30 min.
Read the color of the suspension in the tubes after 30 min. incubation. Strains of N. meningitidis AND N. lactamica may be identified at this time (Figure 1).
If the cell suspension in the tube is blue, the organism is N. lactamica.
If the cell suspension in the tube is yellow, the organism is N. meningitidis.Figure 1. Schematic Diagram of an Enzyme Substrate Test.
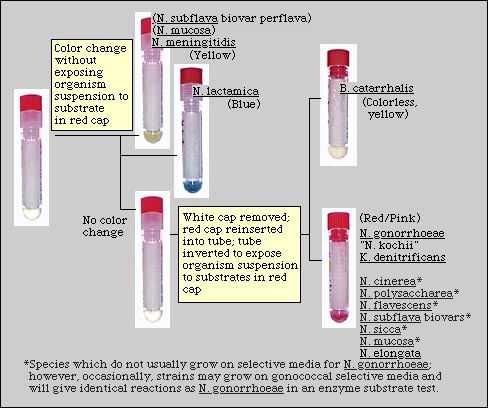
If an identification is made in this step, the test is complete. Do not continue with step 9.
If no color change in the cell suspension has been observed in step 8, the isolate has not been identified; continue with step 9.
If the color of the cell suspension is unchanged, both caps (red and clear) are removed from the tube. The red cap - separated from the clear cap (which is discarded) - is inserted in the tube and the tube is inverted several times to bring the cell suspension in contact with the substrate contained on the bottom side of the red cap (Figure 1). Return the tube to the upright position. The liquid will either turn red-pink or remain colorless or turn pale yellow.
If the cell suspension turns pink-red, the organism produces hydroxyprolylaminopeptidase and may be identified presumptively as N. gonorrhoeae.
If the cell suspension remains unchanged in color or turns a pale yellow, the organism is M. catarrhalis.- Read and record test results.
Quality Control Schedule:
- Quality control tests of Gonochek-II is performed at the time of receipt of the product from the manufacturer.
Problems & Solutions
If QC strains give incorrect reactions, strains may have been mislabeled. Prepare new frozen stocks of QC strains identified by other methods. Confirm reactions of QC strains in the enzyme substrate test at the time the new QC strains are prepared.
Test only oxidase-positive, gram-negative diplococci isolated on selective medium e.g., Martin-Lewis medium. Strains isolated on nonselective media may be tested only if they are confirmed to be colistin resistant by their ability to grow on selective media.
Preservatives in PBS may interfere with enzyme reactions; use only presevative-free PBS.
Do not test organisms which were grown only on nonselective media such as chocolate agar without confirming colistin resistance.
Enzyme substrate tests should not be used as the only test for identifying N. gonorrhoeae, particularly if such an identification may have medicolegal implications.
Some strains of N. mucosa and N. perflava may produce pale yellow reactions suggesting that gamma-glutamylaminopeptidase has been produced. Pigmented strains which produce gamma-glutamylaminopeptidase should be tested to detect acid production patterns before identified as N. meningitidis.
Limitations of Test
The use of Gonochek-II should be limited to the testing of strains of gram-negative, oxidase-positive diplococci isolated on gonococcal selective media or of isolates whose colistin resistance has been confirmed independently.
Because K. denitrificans and several Neisseria and related spp. that give a positive hydroxyprolylaminopeptidase reaction have been isolated on, and may grow on gonococcal selective medium when subcultured, a confirmed identification of N. gonorrhoeae cannot be made based solely on a positive hydroxyprolylaminopeptidase reaction in an enzyme substrate test. Additional tests must be performed before a confirmed identification of N. gonorrhoeae may be made.
Supplemental tests for differentiating between N. gonorrhoeae and related species that produce hydroxyprolylaminopeptidase
Enzyme substrate tests should be used to identify only those strains that grow on selective medium for N. gonorrhoeae. However, strains of K. denitrificans are routinely isolated on selective media and strains of several commensal Neisseria (N. cinerea, N. polysaccharea, N. subflava biovar perflava) have occasionally been isolated on these media. Thus, for the purposes of clarification, the reactions of all Neisseria and related species of human origin are included in this table, together with differential tests which may be used to accurately identify these species.
Table 1. Differential characteristics of human Neisseria and related species that produce hydroxyprolylaminopeptidase in enzyme substrate tests.
| Species that Produce Hydroxy- prolylaminopeptidase | Acid Production Patterns | Gram stain* | Nitrate Reduction | Polysacc- haride from Sucrose | Superoxol | Colistin Resistance | |||
|---|---|---|---|---|---|---|---|---|---|
| G | M | L | S | ||||||
| N. gonorrhoeae "N. kochii"* | + | - | - | - | GND | - | - | Strong (4+) | R |
| K. denitrificans | + | - | - | - | GNC | + | - | - | R |
| N. cinerea | + | - | - | - | GND | - | - | Weak (2+) | (R) |
| N. polysaccharea | + | + | - | - | GND | - | + | Weak (2+) | (R) |
| N. subflava biovars subflava/flava | + | + | - | - | GND | - | - | Weak (2+) | S |
| N. subflava biovar perflava | + | + | - | + | GND | - | - | Weak (2+) positive | S |
| N. sicca | + | + | - | + | GND | - | - | Weak (2+) positive | S |
| N. mucosa | + | + | - | + | GND | + | - | Weak (2+) positive | S |
| N. elongata | - | + | - | - | GNR | - | - | Weak (2+) positive | S |
| N. flavescens | - | - | - | - | GND | - | - | Weak (2+) positive | S |
Abbreviations: G, glucose; M, maltose; L, lactose; S, sucrose; +, most strains positive; -, most strains negative; GND, Gram-negative diplococci; GNC, Gram-negative coccobacilli; GNR, Gram-negative rods; R, colistin-resistant; (R), most strains susceptible, but some strains known to be colistin-resistant; S, most strains susceptible; does not preclude the possibility that some strains may be resistant.
*Cell morphology may be confirmed with a cellular elongation test.
Results, Interpretation, and Reporting
Although enzyme substrate tests are marketed as a confirmatory test for the identification of N. gonorrhoeae isolated on selective medium for the gonococcus, several Neisseria and related species may be isolated on selective media. These species include, most notably but not exclusively, K. denitrificans, N. cinerea, N. polysaccharea, and N. subflava biovar perflava.
The expected results in the Gonochek II test are shown in Figure 1. Confirmed identifications of gram-negative, oxidase-positive diplococci isolated on gonococcal selective media may be made on the basis of an enzyme substrate test for:
- N. lactamica (beta-galactosidase-positive),
- N. meningitidis (gamma-glutamylaminopeptidase-positive), and
- M. catarrhalis (no reaction)
If an isolate is hydroxyprolylaminopeptidase-positive:
Because strains of several Neisseria and related species which may be isolated on gonococcal selective medium produce prolylaminopeptidase, it is only possible to make a presumptive identification of N. gonorrhoeae based on the results of an enzyme substrate test alone. Additional tests that may aid in accurately identifying an isolate are shown in Table 1.
If a report of "Presumptive N. gonorrhoeae" is made on the basis of an identification with an enzyme substrate test alone, it is important that a clinician receiving this report understands that additional tests may be required to confirm this identification.
In view of the potential serious medicolegal and social consequences of reporting an isolate as N. gonorrhoeae, it is advisable to perform additional tests concurrently with, or immediately following, the enzyme substrate test in order to report a confirmed result rather than a presumptive identification which may result in the initiation of investigations of sexual assault or abuse.
Bibliography
D'Amato RF, Enriques LA, Tomforde KN, Singerman E. Rapid identification of Neisseria gonorrhoeae and Neisseria meningitidis by using enzymatic profiles. J Clin Microbiol 1978;7:77-81.
Product Insert. GonochekTM-II tubes, E-Y Laboratories Inc., San Mateo. Welborn PP, Uyeda CT, Ellison-Birang N. Evaluation of GONOCHEK II as a rapid identification system for pathogenic Neisseria species. J Clin Microbiol 1984;20:680-683.
Knapp JS. Historical perspectives and identification of Neisseria and related species. Clin Microbiol Rev 1988;1:415-431.
Knapp JS, Totten PA, Mulks MH, Minshew BH. Characterization of Neisseria cinerea, a non-pathogenic species isolated on Martin-Lewis medium selective for pathogenic Neisseria spp. J Clin Microbiol 1984;19:63-67.
- Page last reviewed: December 10, 2013
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir