Nitrate Reduction Test
Among the human Neisseria and related species, three species -- N. mucosa, M. catarrhalis, and K. denitrificans reduce nitrate. The nitrate reduction test is a critical test for differentiating between N. gonorrhoeae and K. denitrificans, particularly when strains of K. denitrificans appear to be gram-negative diplococci in stained smears.
Principle
Bacterial species may be differentiated on the basis of their ability to reduce nitrate to nitrite or nitrogenous gases. Among the Neisseriaceae of human origin, strains of Neisseria mucosa, Moraxella catarrhalis, and Kingella denitrificans reduce nitrate. Strains of M. catarrhalis and K. denitrificans have been misidentified as N. gonorrhoeae. The nitrate reduction test permits differentiation between these species which are nitrate positive and N. gonorrhoeae (nitrate-negative). The reduction of nitrate may be coupled to anaerobic respiration in some species.
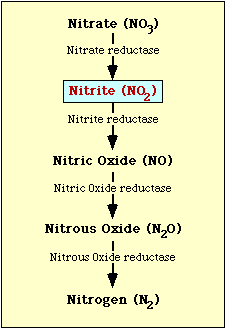
The biochemical pathway involved in nitrate reduction is shown in Figure 1. Nitrate is reduced to nitrite which may then be reduced to nitric oxide, nitrous oxide, or nitrogen (Figure 1).
Figure l. Nitrate reduction pathway.
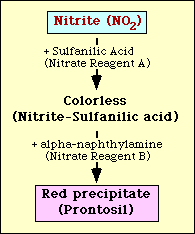
The nitrate reduction test is based on the detection of nitrite in the medium after incubation with an organism. If present in the medium, nitrite will react with sulfanilic acid (Nitrate reagent A) to form a colorless complex (nitrite-sulfanilic acid). This complex will then yield a red precipitate (prontosil) when nitrate reagent B (alpha-naphthylamine) is then added to the test as shown in Figure 2.
Figure 2. Diagrammatic representation of the detection of nitrite in medium.
A red color will be produced in the medium only when nitrite is present in the medium. Lack of a red color in the medium after the addition of sulfanilic acid and alpha-naphthylamine means only that nitrite is not present in the medium. There may be two explanations for this observation.
- The nitrate may not have been reduced; the strain is nitrate-negative.
- The nitrate may have been reduced to nitrite which has then been completely reduced to nitric oxide, nitrous oxide, or nitrogen which will not react with the reagents that react with nitrite; the strain is nitrate-positive.
Any test medium that gives a negative result after the addition of the nitrate reagents must be further tested to determine which of the two interpretations is accurate.
A successful nitrate reduction test is dependent on performing the test under the correct conditions.
- The reaction will occur best if the base medium supports growth of the organism. However, although some Neisseria species do not grow well in broth media, the nitrate reduction test may be performed successfully in a medium that does not support growth by inoculating the medium heavily to provide sufficient preformed enzyme for the reaction to occur.
- Nitrate reaction occurs only under anaerobic conditions. The nitrate-containing medium is dispensed in tubes to give a low surface area:depth ratio which limits the diffusion of oxygen into the medium, e.g., 5 ml of medium is dispensed in a 13 mm diameter tube. Neisseria and related species use the oxygen in the medium and rapidly produce anaerobic conditions which are ideal for the reduction of nitrate to occur.
The nitrate reduction test is performed in a medium containing 0.2% potassium nitrate. The medium is inoculated heavily with a pure culture of the suspect organism and incubated at 35C to 36.5C for 48 h. in an incubator with or without supplemental carbon dioxide.
Nitrate reduction is detected with the Griess Llosvay reagents, sulfanilic acid and alpha-naphthylamine. Sulfanilic acid (Nitrate reagent A) is added to the incubation mixture and forms a complex (nitrite-sulfanilic acid) with any nitrite present in the medium. When alpha-naphthylamine (Nitrate reagent B) is added to the incubated medium, a red precipitate (prontosil) will be formed with any nitrite-sulfanilic acid complex present in the medium.
An organism may be reported as nitrate-positive if a red color develops in the medium after Nitrate reagents A and B are added to the medium, indicating that the organism has reduced nitrate to nitrite.
The absence of a red color after the addition of both reagents does not automatically mean that the organism is unable to reduce nitrate. Strains may have reduced the nitrate to nitrite, and then reduced the nitrite completely to nitrogenous gases which are not detected when Nitrate reagents A and B are added to the medium. If the medium does not change color after the addition of sulfanilic acid and alpha-naphthylamine, a small amount ("knife point") of zinc dust is added to the incubated medium. The zinc dust will catalyze the reduction of nitrate to nitrite chemically. Thus, if the nitrate has not been reduced by the organisms, i.e., they are nitrate-negative, it will be reduced by the zinc dust and a red color will develop in the incubated medium within 15 min. If no color develops in the incubated medium after the addition of zinc dust, the organisms have not only reduced nitrate to nitrite, but have reduced nitrite to nitrogenous gases; these organisms are also nitrate-positive.
Although nitrate medium is supplied with inverted Durham tubes to detect gas production, gas production is not recorded for Neisseria species. Although some species may reduce nitrate beyond nitrite to nitrogenous gases, gas may not accumulate in the tube. The accumulation of gas is dependent on the rate at which it is produced. When gas is produced very slowly, it may dissolve in the medium and not accumulate in the Durham tube.
Specimen Requirements
Optimum specimen: A pure culture of a suspect gram negative, oxidase-positive diplococcus (Neisseria spp. or M. catarrhalis) on chocolate agar incubated in a carbon dioxide-enriched atmosphere at 35C to 36.5C for 18 to 24 h.
Unacceptable specimen: Cultures of isolates on chocolate agar incubated in a carbon dioxide- enriched atmosphere at 35C to 36.5C for more than 24 h.
Compromising factors affecting test results:
- Test medium must be inoculated heavily enough to permit reaction to occur with preformed enzymes. Insufficient inoculum may not permit organisms to use up oxygen to produce anaerobic conditions in which nitrate reduction can occur.
- Too much zinc dust added to the incubated tube may result in very rapid reduction of nitrate beyond nitrite to nitrogenous gases so that nitrite is not detected.
Stability of specimen: Detection of nitrate reduction for Neisseria and related species is dependent on the presence of preformed enzymes.
- Tests should only be performed with inoculum harvested from 24 h cultures.
- Nitrate medium should be inoculated within 30 min. of removing culture from the incubator; prolonged exposure of the culture at room temperature may result in diminished enzyme activity.
Medium/Reagents
Medium: Nitrate broth (Heart infusion broth containing 0.2% potassium nitrate)
Heart infusion broth (Difco), 25.0 g
Potassium nitrate, 2.0 g
Distilled water, 1000.0 ml
- Dissolve the ingredients in distilled water; adjust solution to pH 7.0.
- Dispense 5 ml aliquots of the broth into 16 mm x 100-mm tubes with gas inserts (Durham tubes, 6-mm x 50-mm).
- Autoclave for 15 min at 121C.
Store medium at 4C to 10C (refrigerated) until used. Prewarm the medium to room temperature before inoculation.
Reagents: Sulfanilic acid solution (Nitrate reagent A): 0.8% in 5N acetic acid
Chemical Name: 4-aminobenzene sulfonic acid
Store Nitrate reagent A at 15C to 30C (room temperature) for up to 3 months, in dark. Reagents may be stored in dark brown glass containers; bottles may be wrapped in aluminum foil to ensure darkness.
Alpha-Naphthylamine solution (Nitrate reagent B): 0.6% in 5N acetic acid
Chemical Name: N,N-dimethyl-1 naphthylamine
Store Nitrate reagent B at 2C to 8C (refrigerated) for up to 3 months, in dark. Reagents may be stored in dark brown glass containers; bottles may be wrapped in aluminum foil to ensure darkness.
Zinc powder, Reagent grade: Store at room temperature (15C to 30C)
Warning: Acetic acid is corrosive. Contact with skin may cause blisters and burns. In case of contact, flush eyes and skin immediately with plenty of water (for at least 15 min.)
Quality Control/Test Procedure
QC strains:- Nitrate reductase-positive control: Kingella denitrificans, CDC 10,236
- Nitrate reductase-negative control: Neisseria gonorrhoeae, ATCC 43069
QC strains are stored at -70C in a solution of tryptic soy broth containing 20% glycerol. Reactions of control strains should be confirmed at the time frozen stocks are prepared. QC strains may be stored at -70C for up to 2 years.
Procedure:
QC strains are tested in the same manner as clinical isolates. QC strains should be subcultured at least once after the initial culture from the frozen specimen before the test is performed. Clinical isolates may be subcultured from selective medium or purified subcultures. Ensure that cultures are pure.
- Thaw vials of control strains stored at -70C.
- Streak onto plates of chocolate agar or supplemented GC agar for isolation. Incubate at 35C to 36.5C in a carbon dioxide-enriched atmosphere for 18 to 24 h.
With a sterile swab, prepare a heavy suspension of well-isolated colonies from a pure culture of the isolate incubated on chocolate medium at 35C to 36.5 C in a carbon dioxide-enriched atmosphere for 18 to 24 h. Inoculate test medium to give heavy turbidity.
Note: Strains of N. gonorrhoeae and some other Neisseria spp. may not grow in this medium. Thus, the reaction may be dependent on preformed enzyme.
- Incubate inoculated media and an uninoculated medium control medium tube at 35C to 36.5 C in a carbon dioxide-enriched atmosphere for 48 h.
After 48 h. incubation add, with Pasteur pipettes, 5 drops of Reagent #A, followed by 5 drops of Reagent #B to each tube. Shake the tube well to mix reagents with medium.
Examine the suspension for a pink-red color which should develop within a few minutes if the medium is still warm. The reaction may take a little longer if the medium is cold when the reagents are added.
If the suspension turns pink-red before the addition of Zn powder, the recation is positive and the test is completed. Do not perform step 4.
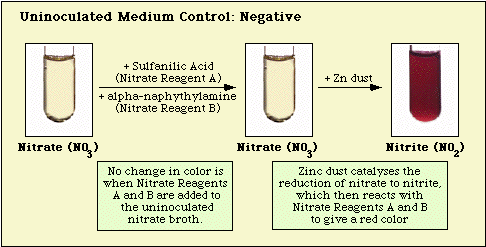
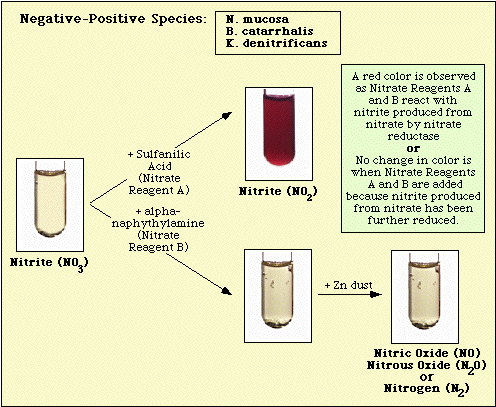
Reactions observed with the uninoculated medium control, and nitrate-negative, and nitrate-positive isolates are illustrated in Figure 3, Figure 4, and Figure 5, respectively.
If the suspension is colorless after the addition of reagents A and B, add a small amount (4 to 5 mg; "sharp knife point") of zinc dust to the medium. Shake the tube vigorously and allow it to stand at room temperature for 10-15 min.
If the medium remains colorless after the addition of Zn powder, the test result is positive.
If the medium turns pink after the addition of Zn powder, the result is negative.
- Read and record results.
Figure 3. Reaction Observed with Uninoculated Nitrate Medium.
Figure 4. Reaction Observed with Nitrate-Negative Species.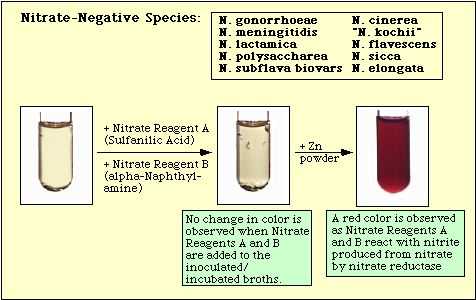
Figure 5. Reactions Observed with Nitrate-Positive Species.
Quality Control Schedule:
- A nitrate reductase QC test is performed each day clinical isolates are tested.
Problems & Solutions
The nitrate reduction test may give false-negative or false positive results if the medium is not produced accurately or the test is not performed accurately. The reaction in this test is dependent upon a number of factors.
- Failure to detect pink color in the uninoculated medium control tube after the addition of Zn dust may be due (1) to the medium not containing nitrate or (2) to the addition of too much zinc dust which has catalyzed the reduction of nitrate beyond nitrite to nitrogenous gases. The simplest solution is to obtain more nitrate medium ensuring that nitrate has been added to the base medium. Alternatively, inoculate the medium with a positive control strain, but test for a reaction after a shorter incubation time; strains of N. mucosa will produce a positive nitrite reaction after a few hours incubation. If the medium is confirmed to contain nitrate, repeat the test until you have determined the correct amount of zinc dust to add. It is critical to learn how much zinc dust to add to the test. Addition of too much zinc dust can result in a false-positive result.
- If a pink color is detected in the uninoculated medium control after nitrate reagents A and B have been added to the medium, the medium is contaminated with nitrite. The only solution is to obtain a new batch of medium that is not contaminated with nitrite.
- In medium containing nitrate, the failure of the positive control strain, Kingella denitrificans, to give a positive reaction would occur only if the strain is not K. denitrificans. Recheck the identity of the positive control strain. Select a new culture of the control strain and repeat the test. Similarly, if a positive nitrate reductase test is obtained with the negative control strain, N. gonorrhoeae, either the negative control strain is not N. gonorrhoeae or the culture is contaminated with a nitrate-positive organism. Recheck the purity and identity of gonococcal reference strain. Repeat test with a pure culture of a confirmed culture of N. gonorrhoeae.
- The nitrate reduction reaction indicates the ability of organisms to reduce nitrate, a reaction that occurs only under anaerobic conditions; the reaction will not occur if the organisms receive a continuous supply of oxygen. Thus, the reaction may not occur in still cultures (particularly of slow-growing species) in which the medium is distributed in shallow layers that permit oxygen to diffuse into the medium. A test to determine if oxygen is present in the medium may be made by adding a drop of oxidase reagent to the medium. If the medium turns purple, the medium contains oxygen and the nitrate reduction reaction may not occur. If the medium remains colorless, the medium does not contain oxygen and the nitrate reduction test may occur. It has been noted that N. gonorrhoeae cells use up the oxygen rapidly if sufficient cells are inoculated into the medium. If oxidase reagent is added after approximately 1 to 2 hr incubation, the medium will remain clear. Because the oxidase reagent kills the gonococci present in the medium, the medium will gradually turn purple, starting at the top of the tube, as the oxygen diffuses into the medium. If the medium is dispensed in tubes of different dimensions from those suggested above, ensure that the surface area-to-depth ratio is at least equal to or smaller than those suggested above. If the diameter of the tube into which the medium is dispensed is larger than that described above, use a larger volume of medium to maintain the same surface area-to-depth ratio.
The nitrate reduction reaction may not occur if the medium in which the test is performed does not permit normal growth of the organism. However, the test may be performed in a medium that does not support growth of the organisms if the inoculum is sufficiently dense that preformed enzymes can exhaust the existing oxygen supply and reduce the nitrate at a faster rate than that at which the oxygen diffuses into the medium.
Note: To check that the oxygen has been removed from the medium, add 2 to 3 drops of oxidase reagent to a duplicate of the inoculated medium. If the oxygen has been adequately removed from the medium, the oxidase reagent will not turn purple immediately. If the medium contains dissolved oxygen, the oxidase reagent will turn purple. Note also that the nitrate reduction test may be performed in medium to which the oxidase reagent has been added.
- When Reagent A is added to the test medium, nitrite produced as a result of the reduction of nitrate will form a complex with the sulfanilic acid that produces a red precipitate with the alpha-naphthylamine in Reagent B. The presence of a red color in the test medium indicates that nitrite is present as a result of the reduction of nitrate. However, the absence of a red color after the addition of Reagents A and B does not necessarily mean that the nitrate was not reduced. The failure to develop a red color may mean (1) that the nitrate was not reduced, or (2) that the nitrite, produced as a result of the reduction of nitrate, has itself been reduced to nitrogenous gases. To determine whether the nitrite has been reduced, place a small quantity of zinc dust in the incubation mixture if it is colorless after the addition of Reagents A and B. The zinc dust catalyzes the reduction of nitrate to nitrite; a red color should develop in the medium which still contains unreduced nitrate. It is important, however, not to add too much zinc dust; excess zinc dust will catalyze the reduction of nitrite produced from that nitrate, resulting in a colorless medium and the incorrect interpretation of the test as positive (a false-positive result).
- A positive nitrate reductase test is obtained with the negative control strain, N. gonorrhoeae, after the addition of the zinc dust indicates that the nitrate has been reduced beyond nitrite, probably due to the addition of too much zinc dust to the test. Repeat the test making sure to add very little zinc dust. The pink color, indicating that the organism has not reduced nitrate, may take 10 to 15 min. to develop. Do not add more zinc dust! Wait for the color to develop. If no color has developed in 30 min., interpret the test as positive.
Limitations of Test
If the test is performed properly and quality control strains give appropriate results, there should be no limitations to this test. Care must be taken to ensure that all components of the test are performed properly .
No identification of genus or species can be made on the basis of the nitrate reduction test alone.
Results, Interpretation, and Reporting
Isolates may be reported as Nitrate-positive if nitrite (pink color) is detected in the inoculated medium after the addition of reagents A and B or if no color is detected in the medium after the addition of zinc dust.
Isolates may be reported as Nitrate-negative if nitrite is not detected (no color change) after the addition of reagents A and B, or if a pink color develops after the addition of zinc dust to the inoculated medium.
Bibliography
Knapp JS, Clark VL. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect Immun 1984;46:176-181.
Skerman VBD. 1967. p.218 - 220. A guide to the identification of the genera of bacteria. The Williams & Wilkins Co., Baltimore, MD.
- Page last reviewed: December 10, 2013
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir