Strain Typing/Serology
A serological typing method for N. gonorrhoeae was developed with monoclonal antibodies in 1984. This typing system was based on the studies of polyvalent antisera-N. gonorrhoeae reactions by several investigators.
Polyvalent Antibody Typing
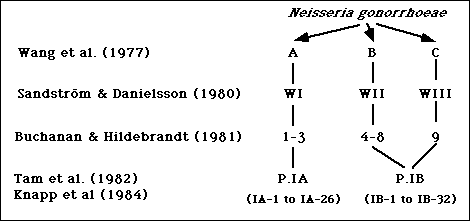
Wang et al. divided gonococcal isolates into three groups, designated A, B, and C, with a microimmunofluorescence test using polyvalent antibodies against formalinized whole gonococcal cells (1). Subsequently, Sandstrom and Danielsson used a coagglutination test to divide gonococci into three serologically distinct groups designated WI, WII, and WIII (2). Serogroups WI, WII, and WIII corresponded to Wang's serogroups A, B, and C, respectively (2). In 1981, Buchanan and Hildebrandt divided gonococcal strains into nine serotypes, numbered 1 to 9, using an enzyme-linked immunosorbent assay (ELISA) with purified gonococcal protein I (PorI) (3). Serotypes 1-3, 4-8, and 9, usually corresponded to serogroups WI, WII, and WIII, respectively (4). A summary of these studies is shown in Figure 1.
Figure 1. Summary of serotyping systems for N. gonorrhoeae based on polyvalent antisera.
Peptide Mapping Studies
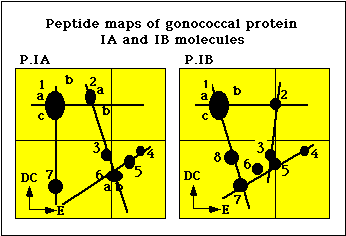
The coagglutination and the ELISA typing systems were shown to detect antigenic determinants on the gonococcal protein I (PorI) molecule (5). Peptide mapping analysis of PI molecules by Sandstrom et al. showed that isolates belonging to the serogroups WII and WIII possessed similar PI molecules, designated PIB, whereas isolates belonging to the serogroup WI possessed a distinctly different PI molecule, designated PIA (Figure 2) (6). Tam et al. developed monoclonal antibodies against epitopes on the gonococcal protein I molecule (7).
Figure 2. Peptide maps of P.IA and P.IB molecules of N. gonorrhoeae.
Monoclonal Antibody Typing
A serotyping system was developed using a standard panel of six PIA-specific and six PIB-specific monoclonal antibody reagents in coagglutination tests (8). Serovars were defined by the patterns of reactions of boiled suspensions of gonococcal isolates with either the PIA- (Table 1) or the PIB-specific (Table 2) reagents in coagglutination tests (8, 9, unpublished data). In a study of strains from a worldwide collection, all strains reacted with at least one of these reagents (8). Currently, 26 PIA serovars, designated IA-1 to IA-26, and 31 PIB serovars, designated IB-1 to IB-31, have been identified (8, 9, unpublished data).
Table 1. Serological classification of Neisseria gonorrhoeae with P.IA-specific monoclonal antibodies in a coagglutination test
| IA-Specific Reagent | IA-Serovar | |||||
|---|---|---|---|---|---|---|
| 4A12 | 4G5 | 2F12 | 6D9 | 5G9 | 5D1 | |
| X | • | • | X | • | • | IA-13 |
| X | X | • | • | • | • | IA-15 |
| X | X | X | • | • | • | IA-14 |
| X | X | X | • | . | X | IA-24 |
| X | • | X | X | • | • | IA-19 |
| X | X | • | X | • | • | IA-7 |
| X | X | • | X | X | • | IA-23 |
| X | X | X | X | • | • | IA-5 |
| X | X | X | • | X | • | IA-11 |
| X | X | X | X | X | • | IA-9 |
| X | X | X | • | • | X | IA-12 |
| X | X | X | X | X | X | IA-1 |
| • | X | X | X | X | X | IA-2 |
| • | X | X | • | X | X | IA-6 |
| • | X | • | X | X | X | IA-20 |
| • | X | X | X | X | • | IA-10 |
| • | X | X | • | X | • | IA-3 |
| • | X | • | • | X | • | IA-25 |
| • | X | X | X | • | • | IA-21 |
| • | X | • | X | • | • | IA-16 |
| • | X | X | • | • | • | IA-8 |
| • | X | • | • | • | • | IA-4 |
| • | • | X | • | • | • | IA-17 |
| • | • | X | X | • | • | IA-22 |
| • | • | X | X | X | X | IA-18 |
| • | • | • | • | X | • | IA-26 |
Abbreviations: X = positive, • = negative.
Table 2. Serological classification of Neisseria gonorrhoeae with P.IB-specific monoclonal antibodies in a coagglutination test
| IB-Specific Reagent | IB-Serovar | |||||
|---|---|---|---|---|---|---|
| 3C8 | 1F5 | 2D6 | 2G2 | 2D4 | 2H1 | |
| X | • | • | • | • | • | IB-20 |
| X | • | • | • | • | X | IB-6 |
| X | • | • | • | X | X | IB-13 |
| X | X | • | • | • | • | IB-14 |
| X | X | • | • | • | X | IB-3 |
| X | X | • | • | X | X | IB-9 |
| X | • | X | • | • | • | IB-16 |
| X | • | X | • | • | X | IB-2 |
| X | • | X | • | X | X | IB-21 |
| X | • | X | X | • | X | IB-31 |
| X | X | X | • | • | • | IB-10 |
| X | X | X | • | • | X | IB-1 |
| X | X | X | X | • | X | IB-26 |
| • | X | • | • | • | • | IB-17 |
| • | X | • | • | • | X | IB-23 |
| • | X | X | • | • | • | IB-25 |
| • | X | X | • | • | X | IB-22 |
| • | X | • | X | • | X | IB-18 |
| • | X | • | X | X | X | IB-11 |
| • | X | • | • | X | X | IB-27 |
| • | X | X | X | • | • | IB-30 |
| • | X | X | X | • | X | IB-5 |
| • | • | X | • | • | • | IB-29 |
| • | • | X | • | • | X | IB-19 |
| • | • | X | X | • | X | IB-7 |
| • | • | X | • | X | X | IB-28 |
| • | • | X | X | X | X | IB-12 |
| • | • | • | X | • | X | IB-8 |
| • | • | • | X | X | X | IB-4 |
| • | • | • | • | X | • | IB-24 |
| • | • | • | • | X | X | IB-15 |
| • | • | • | • | • | X | IB-32 |
Abbreviations: X = positive, • = negative.
Serological Nomenclature
The nomenclature for this serological classification system requires the use of a standard panel of reagents in order to permit the comparison of results of serotyping between different geographical areas and different time periods, considered paramount to follow the spread of gonococcal strains globally (8). At this time, a number of strains have been found not to react with the standard panel of 12 monoclonal antibodies. Two additional monoclonal antibodies which have a broad range of reaction with either IA (9D2) or IB (2H7) have been used to identify the protein I class to which these isolates belong (unpublished data)
Swedish investigators devised a serotyping system using a coagglutination test with the monoclonal antibodies described above (designated GS-antibodies) and additional antibodies (designated Ph-antibodies) (10, 11). In this typing system, each monoclonal antibody reagent in the panels was assigned a lower-case letter designation (10, 11). The serovar nomenclature used in these sytems a combination of upper- and lower-case letters. The serovar of an isolate was designated A or B to indicate that the isolate produced the IA or IB molecule, respectively. Thus, the serovar of an isolate that reacted with the IA-specific monoclonal antibody reagents e, d, i, and h, was named Aedih (11). Swedish investigators used different panels of reagents to provide greater resolution among serovars that were predominant in some patient populations. Unfortunately, a standard panel of antibody reagents was not used for all studies; thus, it is not possible to compare the data from different studies.
Type-specific monoclonal antibodies have also been produced which recognized type-specific epitopes of the serotypes 1, 5, 7, 8 and 9 (12). Serotype 9 has been divided into two subtypes, 9a and 9b. In this system, the serotype of an isolate is designated by its pattern of reaction with individual monoclonal antibody reagents; thus, an isolate that reacts with reagents 5, 7, and 8 in a coagglutination test is designated serotype 5, 7, 8. Although the high degree of resolution of the serovar typing system permits more detailed studies of gonococcal strain populations, the serotyping system, by giving less-detailed analyses, may permit a more practical grouping of antigenically related serovars for studies of antimicrobial susceptibilities. The serotyping reagents are included in a commercially available typing system for gonococcal isolates, but have not been used extensively for studies of the distribution of gonococcal isolates.
References
- Wang SP, Holmes KK, Knapp JS, Ott S, Kyzer. Immunologic classification of Neisseria gonorrhoeae with immunofluorescence. J Immunol 1977;119:794-803.
- Sandstrom EG, Danielsson D. Serology of Neisseria gonorrhoeae. Classification by coagglutination. Acta Pathol Microbiol Scand Sect B 1980;88:27-38.
- Buchanan TM, Hildebrandt JF. Antigen-specific serotyping of Neisseria gonorrheoae characterization based upon principal outer membran protein. Infect Immun 1981;32:895-994.
- Sandstrom EG, Bygdeman S. Serological classification of Neisseria gonorrhoeae. Clinical and epidemiological applications. In. Poolman JT, Zanen HC, Meyer TF, Heckels JE, Makela PRH, Smith H, Beuvery Ec (Ed). Gonococci and meningococci. Kluwer Academic Publishers, Dordrecht, The Netherlands. 1986, 45-50.
- Sandstrom EG, Knapp JS, Buchanan TM. Serology of Neisseria gonorrhoeae. W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun 1982;35:229-239.
- Sandstrom EG, Chen KCS, Buchanan TM. Serology of Neisseria gonorrhoeae: Coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun 1982;38:462-470.
- Tam MR, Buchanan TM, Sandstrom EG, Holmes KK, Knapp JS, Siadak AW, Nowinski RC. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun 1982;36:1042-1053.
- Knapp JS, Tam MR, Nowinski RC, Holmes KK, Sandstrom EG. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis 1984;150:44-48.
- Sarafian SK, Knapp JS. Molecular epidemiology of gonorrhea. Clin Microbiol Rev 1989;2 (Suppl):S49-S55.
- Bygdeman S, Danielsson D, Sandstrom EG. Gonococcal serogroups in Scandinavia. A study with polyclonal and monoclonal antibodies. Acta Pathol Immunol Scand Sect B 1983;91:293-305.
- Danielsson D, Sandstrom E, Bygdeman S, Backman M, Gnarpe H. W-serogroup (protein I) and serovar patterns of gonococci isolated during two different periods in urban and rural districts of Sweden. In. Schoolnik GK, Brooks GF, Falkow S, Frasch CE, Knapp JS, McCutchan JA, Morse SA (ed). The pathogenic neisseriae. American Society for Microbiology, Washington, D. C. 1985, 71-77.
- Kohl PK, Buchanan TM. Serotype-specific bactericidal activity of monoclonal antibodies to protein I of Neisseria gonorrhoeae. In. Schoolnik GK, Brooks GF, Falkow S, Frasch CE, Knapp JS, McCutchan JA, Morse SA (ed). The pathogenic neisseriae. American Society for Microbiology, Washington, D. C. 1985, 442-444.
- Page last reviewed: March 31, 2017
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir