Respiratory alkalosis
Respiratory alkalosis is a medical condition in which increased respiration elevates the blood pH beyond the normal range (7.35–7.45) with a concurrent reduction in arterial levels of carbon dioxide.[1][3] This condition is one of the four basic categories of disruption of acid–base homeostasis.
| Respiratory alkalosis | |
|---|---|
| Other names | Alkalosis - respiratory[1] |
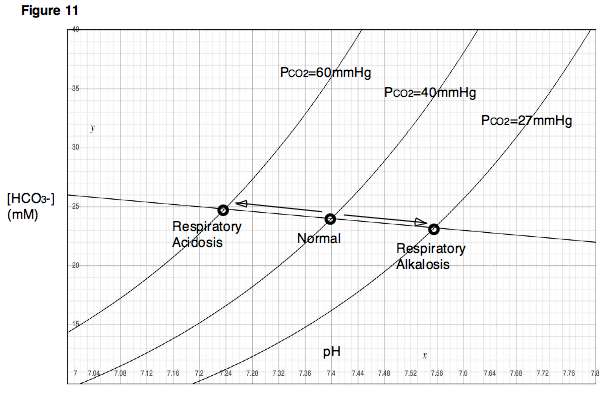 | |
| Davenport diagram outlines pH and bicarbonate levels | |
| Specialty | Endocrinology |
| Causes | Hyperventilation,[1] Pulmonary disorder[2] |
| Diagnostic method | Chest x-ray, Pulmonary function tests[1] |
| Treatment | Detect underlying cause[1] |
Signs and symptoms
Signs and symptoms of respiratory alkalosis are as follows:[4]
Causes
Respiratory alkalosis may be produced as a result of the following causes:
- Stress[1]
- Pulmonary disorder[2]
- Thermal insult[5]
- High altitude areas[6]
- Salicylate poisoning (aspirin overdose)[6]
- Fever[1]
- Hyperventilation (due to heart disorder or other, including improper mechanical ventilation)[1][7]
- Vocal cord paralysis (compensation for loss of vocal volume results in over-breathing/breathlessness).[8]
- Liver disease[6]
Mechanism
The mechanism of respiratory alkalosis generally occurs when some stimulus makes a person hyperventilate. The increased breathing produces increased alveolar respiration, expelling CO2 from the circulation. This alters the dynamic chemical equilibrium of carbon dioxide in the circulatory system. Circulating hydrogen ions and bicarbonate are shifted through the carbonic acid (H2CO3) intermediate to make more CO2 via the enzyme carbonic anhydrase according to the following reaction: This causes decreased circulating hydrogen ion concentration, and increased pH (alkalosis).[9][10]
Diagnosis
The diagnosis of respiratory alkalosis is done via test that measure the oxygen and carbon dioxide levels (in the blood), chest x-ray and a pulmonary function test of the individual.[1]
The Davenport diagram allows clinicians or investigators to outline blood bicarbonate concentrations (and blood pH) after a respiratory or metabolic acid-base disturbance[11]
Treatment
Respiratory alkalosis is very rarely life-threatening, though pH level should not be 7.5 or greater. The aim in treatment is to detect the underlying cause. When PaCO2 is adjusted rapidly in individuals with chronic respiratory alkalosis, metabolic acidosis may occur.[2] If the individual is on a mechanical ventilator then preventing hyperventilation is done via monitoring ABG levels.[15]
Society
In The Andromeda Strain, one of the characters is exposed to contamination, but saves himself by increasing his breathing rhythm until he has respiratory alkalosis in his blood.[16]
See also
- Acidosis
- Alkalosis
- Arterial blood gas
- Chemical equilibrium
- Hypocalcemia
- Metabolic acidosis
- Metabolic alkalosis
- pCO2
- pH
- pKa
- Respiratory acidosis
References
- "Respiratory alkalosis: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2016-02-12.
- "Respiratory Alkalosis: Background, Pathophysiology, Epidemiology". 2018-10-04. Cite journal requires
|journal=(help) - Singh, Virendra; Khatana, Shruti; Gupta, Pranav (2013-01-01). "Blood gas analysis for bedside diagnosis". National Journal of Maxillofacial Surgery. 4 (2): 136–141. doi:10.4103/0975-5950.127641. ISSN 0975-5950. PMC 3961885. PMID 24665166.
- Porth, Carol (2011-01-01). Essentials of Pathophysiology: Concepts of Altered Health States. Lippincott Williams & Wilkins. p. 205. ISBN 9781582557243.
- Feld, Leonard G.; Kaskel, Frederick J. (2009-12-15). Fluid and Electrolytes in Pediatrics: A Comprehensive Handbook. Springer Science & Business. p. 280. ISBN 9781603272254.
- "Alkalosis: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2016-02-12.
- "Hyperventilation: MedlinePlus Medical Encyclopedia".
- "Medscape: Medscape Access". Medscape. 2018-04-05.
- "Evaluation of respiratory alkalosis". us.bestpractice.bmj.com. Retrieved 2016-02-12.
- Wilkins, Lippincott Williams & (2005-01-01). Pathophysiology: A 2-in-1 Reference for Nurses. Lippincott Williams & Wilkins. p. 102. ISBN 9781582553177.
- Albert, Richard K.; Spiro, Stephen G.; Jett, James R. (2008-01-01). Clinical Respiratory Medicine. Elsevier Health Sciences. p. 128. ISBN 978-0323048255.
- Adrogué, HJ; Madias, NE (June 2010). "Secondary responses to altered acid-base status: the rules of engagement". Journal of the American Society of Nephrology. 21 (6): 920–3. doi:10.1681/ASN.2009121211. PMID 20431042.
- Metheny, Norma M. (2011-01-07). Fluid and Electrolyte Balance. Jones & Bartlett Publishers. p. 148. ISBN 9780763781644.
- Klingensmith, Mary E. (2015-10-28). The Washington Manual of Surgery. Lippincott Williams & Wilkins. p. 87. ISBN 9781496310798.
- Handbook of Medical-surgical Nursing. Lippincott Williams & Wilkins. 2006-01-01. p. 801. ISBN 9781582554457.
- "AFI: Robert Wise – Andromeda Strain".
Further reading
- Lang, Florian (2009-03-19). Encyclopedia of Molecular Mechanisms of Disease: With 213 Tables. Springer Science & Business Media. ISBN 9783540671367.
- Unwin, R.; Stidwell, R.; Taylor, S.; Capasso, G. (1997-11-01). "The effects of respiratory alkalosis and acidosis on net bicarbonate flux along the rat loop of Henle in vivo". American Journal of Physiology. Renal Physiology. 273 (5): F698–F705. doi:10.1152/ajprenal.1997.273.5.F698. ISSN 1931-857X. PMID 9374832.
- LeBlanc, P J; Parolin, M L; Jones, N L; Heigenhauser, G J F (2002-10-01). "Effects of respiratory alkalosis on human skeletal muscle metabolism at the onset of submaximal exercise". The Journal of Physiology. 544 (Pt 1): 303–313. doi:10.1113/jphysiol.2002.022764. ISSN 0022-3751. PMC 2290561. PMID 12356901.
External links
| Classification | |
|---|---|
| External resources |