Favipiravir
Favipiravir, also known as T-705 or Avigan, is an experimental antiviral drug being developed by Toyama Chemical of Japan with activity against many RNA viruses. Like some other experimental antiviral drugs (T-1105 and T-1106), it is a pyrazinecarboxamide derivative. Favipiravir is active against influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses.[1] Activity against enteroviruses[2] and Rift Valley fever virus has also been demonstrated.[3] Favipiravir showed limited efficacy against Zika virus in animal studies, but was less effective than other antivirals such as MK-608.[4] The agent has also shown some efficacy against rabies,[5] and has been used experimentally in some humans infected with the virus.[6]
 | |
| Names | |
|---|---|
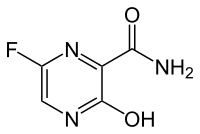
| IUPAC name
5-Fluoro-2-hydroxypyrazine-3-carboxamide | |
| Other names
T-705; Avigan | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C5H4FN3O2 |
| Molar mass | 157.104 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The mechanism of its actions is thought to be related to the selective inhibition of viral RNA-dependent RNA polymerase.[7] Other research suggests that favipiravir induces lethal RNA transversion mutations, producing a nonviable viral phenotype.[8] Favipiravir is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5'-triphosphate (favipiravir-RTP), available in both oral and intravenous formulations.[9][10] Human hypoxanthine guanine phosphoribosyltransferase (HGPRT) is believed to play a key role in this activation process.[11] Favipiravir does not inhibit RNA or DNA synthesis in mammalian cells and is not toxic to them.[1] In 2014, favipiravir was approved in Japan for stockpiling against influenza pandemics.[12] However, favipiravir has not been shown to be effective in primary human airway cells, casting doubt on its efficacy in influenza treatment.[13]
Ebola virus disease
The drug appears to be effective in a mouse model of Ebola virus disease, but its efficacy against human Ebola infection is unproved.[14][15][16] During the 2014 West Africa Ebola virus outbreak, it was reported that a French nurse who contracted Ebola while volunteering for MSF in Liberia recovered after receiving a course of favipiravir.[17] A clinical trial investigating the use of favipiravir against Ebola virus disease was started in Guéckédou, Guinea, during December 2014.[18] Preliminary results showed a decrease in mortality rate in patients with low-to-moderate levels of Ebola virus in the blood, but no effect on patients with high levels of the virus, a group at a higher risk of death.[19] The trial design has been criticised by Scott Hammer and others for using only historical controls.[20] The results of this clinical trial were presented in February 2016 at the annual Conference on Retroviruses and Opportunistic Infections (CROI) by Daouda Sissoko[21] and published on March 1, 2016 in PLOS Medicine.[22]
References
- Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD (June 2009). "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections". Antiviral Research. 82 (3): 95–102. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599.
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (November 2013). "Favipiravir (T-705), a novel viral RNA polymerase inhibitor". Antiviral Research. 100 (2): 446–54. doi:10.1016/j.antiviral.2013.09.015. PMC 3880838. PMID 24084488.
- Caroline AL, Powell DS, Bethel LM, Oury TD, Reed DS, Hartman AL (April 2014). "Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever". PLoS Neglected Tropical Diseases. 8 (4): e2790. doi:10.1371/journal.pntd.0002790. PMC 3983105. PMID 24722586.
- Mumtaz N, van Kampen JJ, Reusken CB, Boucher CA, Koopmans MP (2016). "Zika Virus: Where Is the Treatment?". Current Treatment Options in Infectious Diseases. 8 (3): 208–211. doi:10.1007/s40506-016-0083-7. PMC 4969322. PMID 27547128.
- Yamada K, Noguchi K, Komeno T, Furuta Y, Nishizono A (April 2016). "Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis". The Journal of Infectious Diseases. 213 (8): 1253–61. doi:10.1093/infdis/jiv586. PMC 4799667. PMID 26655300.
- Murphy J, Sifri CD, Pruitt R, Hornberger M, Bonds D, Blanton J, Ellison J, Cagnina RE, Enfield KB, Shiferaw M, Gigante C, Condori E, Gruszynski K, Wallace RM (January 2019). "Human Rabies - Virginia, 2017". MMWR. Morbidity and Mortality Weekly Report. 67 (5152): 1410–1414. doi:10.15585/mmwr.mm675152a2. PMC 6334827. PMID 30605446.
- Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J (2013). "The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase". PLOS ONE. 8 (7): e68347. Bibcode:2013PLoSO...868347J. doi:10.1371/journal.pone.0068347. PMC 3707847. PMID 23874596.
- Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA (April 2013). "T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro". Journal of Virology. 87 (7): 3741–51. doi:10.1128/JVI.02346-12. PMC 3624194. PMID 23325689.
- Guedj J, Piorkowski G, Jacquot F, Madelain V, Nguyen TH, Rodallec A, et al. (March 2018). "Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques". PLoS Medicine. 15 (3): e1002535. doi:10.1371/journal.pmed.1002535. PMC 5870946. PMID 29584730.
- Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, Furuta Y (October 2009). "Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells". The Journal of Antimicrobial Chemotherapy. 64 (4): 741–6. doi:10.1093/jac/dkp274. PMC 2740635. PMID 19643775.
- Naesens L, Guddat LW, Keough DT, van Kuilenburg AB, Meijer J, Vande Voorde J, Balzarini J (October 2013). "Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir)". Molecular Pharmacology. 84 (4): 615–29. doi:10.1124/mol.113.087247. PMID 23907213.
- Koons C (7 August 2014). "Ebola Drug From Japan May Emerge Among Key Candidates". Bloomberg.com.
- Yoon JJ, Toots M, Lee S, Lee ME, Ludeke B, Luczo JM, et al. (August 2018). "Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses". Antimicrobial Agents and Chemotherapy. 62 (8): e00766–18. doi:10.1128/AAC.00766-18. PMC 6105843. PMID 29891600.
- Gatherer D (August 2014). "The 2014 Ebola virus disease outbreak in West Africa". The Journal of General Virology. 95 (Pt 8): 1619–24. doi:10.1099/vir.0.067199-0. PMID 24795448.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S (May 2014). "Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model". Antiviral Research. 105: 17–21. doi:10.1016/j.antiviral.2014.02.014. PMID 24583123.
- Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS (April 2014). "Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model". Antiviral Research. 104: 153–5. doi:10.1016/j.antiviral.2014.01.012. PMID 24462697.
- "First French Ebola patient leaves hospital". Reuters. 4 October 2016.
- "Guinea: Clinical Trial for Potential Ebola Treatment Started in MSF Clinic in Guinea". AllAfrica - All the Time. Retrieved 28 December 2014.
- Fink S (4 February 2015). "Ebola Drug Aids Some in a Study in West Africa". The New York Times.
- Cohen J (26 February 2015). "Results from encouraging Ebola trial scrutinized". Science. doi:10.1126/science.aaa7912. Retrieved 21 January 2016.
- "Favipiravir in Patients with Ebola Virus Disease: Early Results of the JIKI trial in Guinea | CROI Conference". www.croiconference.org. Retrieved 2016-03-17.
- Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. (March 2016). "Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea". PLoS Medicine. 13 (3): e1001967. doi:10.1371/journal.pmed.1001967. PMC 4773183. PMID 26930627.