Ledipasvir/sofosbuvir
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C.[1] It is a combination of ledipasvir and sofosbuvir.[1] Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1.[2] Some evidence also supports use in HCV genotype 3 and 4.[2] It is taken daily by mouth for 8–24 weeks.[1]
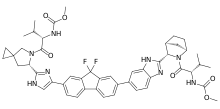 Ledipasvir | |
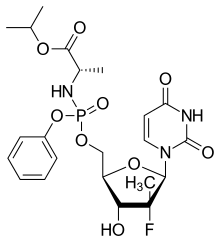 Sofosbuvir structure | |
| Combination of | |
|---|---|
| Ledipasvir | NS5A inhibitor |
| Sofosbuvir | NS5B (RNA polymerase) inhibitor |
| Clinical data | |
| Trade names | Harvoni, Hepcinat-LP, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| KEGG | |
| Chemical and physical data | |
| Formula | C71H83F3N11O15P |
| Molar mass | 1418.476 g/mol |
| Melting point | 170–225 °C (338–437 °F) |
It is generally well tolerated.[3] Common side effects include muscle pains, headache, nausea, rash, and cough.[1] It is unclear if use in pregnancy is safe for the baby.[1] Ledipasvir works by decreasing the activity of NS5A and sofosbuvir works by decreasing the activity of NS5B polymerase.[1]
Ledipasvir/sofosbuvir was approved for medical use in the United States in 2014.[1] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[4] The wholesale cost in the United States is about US$91,589.40 for 12 weeks as of 2016.[5] In Bangladesh this amount costs US$1,092.00.[6] Some people travel to India to get access to lower cost medication.[7]
Medical uses
Cure rates are 94% to 99% in people infected with genotype 1 (46% of HCV cases).[8] It has also been evaluated for the treatment of infection with other hepatitis C genotypes, and has shown promising results in genotypes 3 and 4 (making up 30% and less than 22% of HCV cases respectively).[2][9][10][8]
Resistance
NS5A mutations
Multiple mutations of HCV replicons are necessary to cause a significant effect in resistance due to multiple mechanisms of action.[11] In general, HCV genotype 1a is less resistant to mutation than genotype 1b.[12]
For genotype 1b a single amino acid substitution (e.g. L31V) in the replicon had less than a 100 fold increase in resistance to the ledipasvir in Harvoni, while a two amino acid substitution had over a 1000 fold increase in resistance.[12][13] Genotype 1a had a similar but more substantial increase in resistance with each respective increase in amino acid substitution with resistance associated substitutions at K24R, M28T/V, Q30R/H/K/L, L31M, and or Y93H/N.[14]
NS5A polymorphisms also have an effect on viral resistance with the most common resistance-associated amino acid substitutions detected at Q30R, Y93H or N, and L31M in patients with a rapid virological response (RVR).[14] The specific baseline NS5A resistance-associated polymorphisms observed in clinical trials were M28T/V, Q30H, Q30R, L31M, H58P, Y93H, and Y93N in genotype 1a and L28M, A92T, and Y93H in genotype 1b.[14] Patients with multiple baseline NS5A polymorphisms tend to have higher relapse rates when using ledipasvir/sofosbuvir.[14] The difference in relapse rates between treatment naive and treatment experience groups with baseline NS5A polymorphisms ranges from 1% after a 12-week regimen and 0% after a 24-week regimen respectively.[12][14]
Side effects
More than 10% of people taking ledipasvir/sofosbuvir have headaches or fatigue; rashes, nausea, diarrhea, and insomnia occur in between 1% and 10% of people taking it.[9][17]
More severe reactions are connected with allergic reactions to the medications and cardiovascular problems. Harvoni side effects are considered relatively mild compared to older interferon-based treatment.
Drug interactions
Ledipasvir/sofosbuvir is a substrate for the drug transporters P-Glycoprotein (P-gp) and breast cancer resistance protein (BCRP).[12] Intestinal absorption of these drug transporter substrates may be decreased by inducers such as rifampin and St. John's wort .[18]
Patients are also advised to stay away from H2 Receptor Antagonists (H2RA) and Proton Pump Inhibitors (PPI) because they decrease the concentration of ledipasvir (its solubility is pH-dependent and is higher under acidic conditions). Therefore, it is advised to take a PPI at least two hours after ledipasvir/sofosbuvir with a dose less than or equal to 20 mg daily and H2RAs with a dose of less than or equal to 40 mg twice daily.[12][19]
Ledipasvir/sofosbuvir should additionally be avoided when taking amiodarone or other drugs that lower heart rate; there is a serious risk of the heart slowing or stopping when ledipasvir/sofosbuvir is used with such drugs.[9][17]
Mechanisms of action
The most commonly associated mechanism associated with ledipasvir/sofosbuvir is the hyperphosphorylation of NS5A, a viral polymerase important in proper viral assembly and interferes with proper liver metabolism.[20] Ledipasvir/sofosbuvir inhibits the proper viral assembly by re-positioning NS5A's sub-cellular localization.[12]
NS5B, a viral polymerase that can initiate RNA synthesis de novo, is also allosterically inhibited by ledipasvir/sofosbuvir.[21]
NS5A and NS5B inhibitors in combination have a synergistic effect.[22]
Pharmacokinetics
Sofosbuvir is absorbed fast in the plasma with a peak concentration (Cmax) at 0.8 to 1 hour after the administered dosage and undergoes extra hepatic metabolism with 61 to 65% bound to human plasma proteins.[23][12] It is then predominantly converted to the inactive phosphate free circulating metabolite GS-331007 (eliminated 76% through renal passive filtration) which has a median peak plasma concentration at 3.5 to 4 hours after the medication is ingested.[24][14] Sofosbuvir does not appear to be affected by different levels of macronutrients when compared with fasting states.[25]
Ledipasvir has a maximum concentration at 4 to 4.5 hours after ingestion and is not affected by macronutrients.[12][14] It is more than 98% protein bound and is predominantly eliminated fecally, with minimal metabolism in the liver.[14]
Elimination
The median terminal half life after a dosage of ledipasvir/sofosbuvir for 90 mg of [14C]-Ledipasvir is 47 hours; for 400 mg of [14C]-Sofosbuvir it is 0.5 hours (after the initial distribution of medication in body tissue) and 27 hours (the eventual excretion of the medication).[14][26]
| Substance | ng/mL |
|---|---|
| Ledipasvir | 323 |
| Sofosbuvir | 618 |
| GS-331007 | 707 |
Note: The maximum concentration is 32% higher in healthy individuals than those infected with Hepatitis C.[14]
| Substance | ng*hr/mL |
|---|---|
| Ledipasvir | 7290 |
| Sofosbuvir | 1320 |
| GS-33107 | 12,000 |
Note: The maximum concentration is 24% higher in healthy individuals than those infected with Hepatitis C.[14]
Blood detection
An analytical method based on LC tandem MS has been developed for the simultaneous extraction and determination of ledipasvir/sofosbuvir in human plasma using antiviral daclatasvir as an internal standard. Average extraction recoveries for sofosbuvir and ledipasvir were 91.61% and 88.93% respectively.[27]
Society and culture
One manufacturer is Gilead Sciences.[1]
References
- "Ledipasvir and Sofosbuvir". The American Society of Health-System Pharmacists. Archived from the original on 25 December 2016. Retrieved 8 December 2016.
- Keating GM (2015). "Ledipasvir/Sofosbuvir: a review of its use in chronic hepatitis C". Drugs. 75 (6): 675–85. doi:10.1007/s40265-015-0381-2. PMID 25837989.
- The selection and use of essential medicines: Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children) (PDF). WHO. 2015. p. 70. ISBN 9789240694941. Archived (PDF) from the original on 20 December 2016. Retrieved 8 December 2016.
- "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- "NADAC as of 2016-12-21 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 24 December 2016. Retrieved 25 December 2016.
- Azam, Monirul (2016-05-30). "1". Intellectual Property and Public Health in the Developing World. Open Book Publishers. ISBN 9781783742318. Archived from the original on 2016-12-26.
- "Hep C drug tourism has begun as patients seek Harvoni, Sovaldi overseas". FiercePharma. 2015-06-02. Archived from the original on 2015-11-04. Retrieved 2015-10-25.
- Messina, Jane P.; Humphreys, Isla; Flaxman, Abraham; Brown, Anthony; Cooke, Graham S.; Pybus, Oliver G.; Barnes, Eleanor (January 2015). "Global distribution and prevalence of hepatitis C virus genotypes". Hepatology. 61 (1): 77–87. doi:10.1002/hep.27259. ISSN 1527-3350. PMC 4303918. PMID 25069599.
- "US Label" (PDF). FDA. June 2016. Archived (PDF) from the original on 2017-02-05.. See FDA index page for NDA 205834 Archived 2017-02-05 at the Wayback Machine.
- Canadian Agency for Drugs and Technologies in Health (16 January 2015). "Holkira (Ombitasvir/Paritaprevir/ Ritonavir with Dasabuvir) and Harvoni (Ledipasvir/Sofosbuvir) for Chronic Hepatitis C: A Review of the Clinical Evidence". Rapid Response Service. Canadian Agency for Drugs and Technologies in Health. PMID 25674658. Archived from the original on 5 November 2017.
- Issur, Moheshwarnath; Götte, Matthias (2014-11-06). "Resistance patterns associated with HCV NS5A inhibitors provide limited insight into drug binding". Viruses. 6 (11): 4227–4241. doi:10.3390/v6114227. ISSN 1999-4915. PMC 4246218. PMID 25384189.
- Gritsenko, Diana; Hughes, Gregory (April 2015). "Ledipasvir/Sofosbuvir (Harvoni): Improving Options for Hepatitis C Virus Infection". Pharmacy and Therapeutics. 40 (4): 256–276. ISSN 1052-1372. PMC 4378517. PMID 25859119.
- Gao, Min (October 2013). "Antiviral activity and resistance of HCV NS5A replication complex inhibitors". Current Opinion in Virology. 3 (5): 514–520. doi:10.1016/j.coviro.2013.06.014. ISSN 1879-6265. PMID 23896281.
- "HARVONI (ledipasvir and sofosbuvir)" (PDF). Retrieved 2018-02-22.
- Vermehren, Johannes; Sarrazin, Christoph (August 2012). "The role of resistance in HCV treatment". Best Practice & Research. Clinical Gastroenterology. 26 (4): 487–503. doi:10.1016/j.bpg.2012.09.011. ISSN 1532-1916. PMID 23199507.
- "harvoni_pi.pdf" (PDF). Retrieved 2018-02-21.
- "Harvoni 90 mg/400 mg film-coated tablets - Summary of Product Characteristics". UK Electronic Medicines Compendium. December 2016. Archived from the original on 27 October 2016. Retrieved 4 February 2017.
- "Drug Interactions Between Direct-Acting anti-HCV Antivirals Sofosbuvir and Ledipasvir and HIV Antiretrovirals". www.natap.org. Retrieved 2018-02-22.
- "Impact of Food and Antacids on Levels of Ledipasvir and Sofosbuvir". www.natap.org. Retrieved 2018-02-22.
- Gong, Guozhong; Waris, Gulam; Tanveer, Rasheeda; Siddiqui, Aleem (2001-08-14). "Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-κB". Proceedings of the National Academy of Sciences of the United States of America. 98 (17): 9599–9604. doi:10.1073/pnas.171311298. ISSN 0027-8424. PMC 55498. PMID 11481452.
- Lin, M. Valerie; Chung, Raymond (2014). "Recent FDA approval of sofosbuvir and simeprevir. Implications for current HCV treatment". Clinical Liver Disease. 3 (3): 65–68. doi:10.1002/cld.332. PMC 6448702. PMID 30992888.
- Pawlotsky, Jean-Michel (August 2013). "NS5A inhibitors in the treatment of hepatitis C". Journal of Hepatology. 59 (2): 375–382. doi:10.1016/j.jhep.2013.03.030. ISSN 1600-0641. PMID 23567084.
- "Sofosbuvir". www.drugbank.ca. Retrieved 2018-03-23.
- McQuaid, Thomas; Savini, Carolyn; Seyedkazemi, Star (March 2015). "Sofosbuvir, a Significant Paradigm Change in HCV Treatment". Journal of Clinical and Translational Hepatology. 3 (1): 27–35. doi:10.14218/JCTH.2014.00041. ISSN 2225-0719. PMC 4542085. PMID 26357632.
- Cada, Dennis J.; Baker, Danial E.; Bindler, Ross Jason (March 2015). "Ledipasvir/Sofosbuvir". Hospital Pharmacy. 50 (3): 224–234. doi:10.1310/hpj5003-224. ISSN 0018-5787. PMC 4567193. PMID 26405313.
- "Pharmacology: Basic Pharmacology, ANS, Endocrine". www.kumc.edu. Retrieved 2018-03-23.
- Elkady, Ehab F.; Aboelwafa, Ahmed A. (2016-09-01). "A Rapid and Optimized LC-MS/MS Method for the Simultaneous Extraction and Determination of Sofosbuvir and Ledipasvir in Human Plasma". Journal of AOAC International. 99 (5): 1252–1259. doi:10.5740/jaoacint.16-0021. PMID 27480956.