Dasabuvir
Dasabuvir, sold under the trade name Exviera, is an antiviral medication for the treatment of hepatitis C.[1] It is often used together with the combination medication ombitasvir/paritaprevir/ritonavir specifically for hepatitis C virus (HCV) type 1.[1] Ribavirin may also additionally be used.[2] These combinations result in a cure in more than 90% of people.[3] It is taken by mouth twice a day for 12 to 24 weeks.[1]
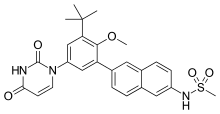 | |
| Clinical data | |
|---|---|
| Trade names | Exviera |
| Other names | ABT-333 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.232.246 |
| Chemical and physical data | |
| Formula | C26H27N3O5S |
| Molar mass | 493.58 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Common side effects include trouble sleeping, nausea, itchiness, and feeling tired.[3] It is not recommended in those with liver failure but appears okay in people with kidney disease.[1] While there is no evidence of harm if used during pregnancy, it has not been well studied.[1] It should not be used with birth control pills that contain ethinylestradiol.[3] Dasabuvir is in the HCV NS5B polymerase inhibitor class of medication.[1]
Dasabuvir was approved for medical use in 2014.[4] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[5] In the United States, it is approved by the Food and Drug Administration only for use in combination with ombitasvir/paritaprevir/ritonavir.[1] In the United States, this combination sold as Viekira Pak, costs 83,319 USD for 12 weeks and 166,638 USD for 24 weeks.[2] As of 2015 the ability to get these medications in many areas of the world is poor.[6]
Medical uses
Dasabuvir is used in the treatment of chronic Hepatitis C infection. It is used in the following HCV subtypes: genotype 1a, genotype 1b, genotype 1 of unknown subtype, and genotype 1 mixed infection without cirrhosis or with compensated cirrhosis.[7]
Contraindications
People should not be taking dasabuvir if they meet any of the following criteria:
- They have a hypersensitivity to it or any of the substances in the tablet.[3]
- They are taking any hormonal contraception that contain ethinylestradiol (often found in combined oral contraceptives or vaginal rings).[3]
- They are also taking medications that are strong or moderate enzyme inducers such as carbamazepine, phenytoin, phenobarbital, efavirenz, nevirapine, etravirine, mitotane, rifampicin, enzalutamide, and St. John's Wort (Hypercium perforatum).[3]
- They are also taking medications that are strong CYP2C8 inhibitors (gemfibrozil).[3]
- They meet contraindication criteria for ombitasivir, paritaprevir, and ritonavir since dasabuvir is used in combination with those three medications.[3]
Adverse effects
The FDA approved combination of dasabuvir used with ombitasvir, paritaprevir, and ritonavir in the product Viekira Pak can cause a number of adverse effects. When Viekira Pak was used without Ribavirin, nausea, severe itching, and insomnia occurred in more than 5% of the subjects.[8] Less commonly, patients experienced increases in liver enzymes, such as AST and ALT, to greater than five times the upper limit of normal (occurred in 1% of patients).[8] Usually this was asymptomatic. However, this is notable because females who are taking ethinylestradiol are at an increased risk for this side effect (25%).[8]
Mechanism of action
Dasabuvir works by inhibiting the action of NS5B palm polymerase, effectively terminating RNA polymerization and stopping the replication of the HCV's genome.[9] By blocking NS5B polymerase, the virus can no longer multiply and infect new cells.[3]
History
The U.S. FDA approved regimen of ombitasvir-paritaprevir-ritonavir and dasabuvir on December 19, 2014 to be used in the treatment of genotype 1 chronic hepatitis C infection in adults, which includes those with compensated cirrhosis.[2]
Administration and storage
The two tablets of ombitasvir, paritaprevir, ritonavir will be taken in the morning and the one dasabuvir tablet taken twice a day in the morning and in the evening with a meal.[8]
The combination pack is packaged in a monthly package for 28 days of treatment.[8] Be sure to store the package and drugs included at or below 30 °C to maintain the integrity of the drugs.[8]
References
- "Viekira Pak". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- "Ombitasvir-Paritaprevir-Ritonavir and Dasabuvir (Viekira Pak) - Treatment - Hepatitis C Online". www.hepatitisc.uw.edu. Archived from the original on 2016-11-01. Retrieved 2016-11-09.
- "European Medicines Agency - Find medicine - Exviera". www.ema.europa.eu. 24 May 2016. Archived from the original on 10 November 2016. Retrieved 2016-11-09.
- Časar, Zdenko (2016). Synthesis of Heterocycles in Contemporary Medicinal Chemistry. Springer. p. 92. ISBN 9783319399171. Archived from the original on 2016-12-20.
- "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- The selection and use of essential medicines: Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children) (PDF). World Health Organization. 2015. p. 73. ISBN 9789241209946. Archived (PDF) from the original on 20 December 2016. Retrieved 10 December 2016.
- Commissioner, Office of the. "Safety Information - Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets), Copackaged for Oral Use". www.fda.gov. Archived from the original on 2016-11-17. Retrieved 2016-11-17.
- AbbVie Inc. (December 2014). "Prescribing Information: Veikira Pak" (PDF). FDA. Archived (PDF) from the original on January 18, 2017. Retrieved October 28, 2016.
- "The DrugBank database". www.drugbank.ca/drugs/DB09183. Archived from the original on 2016-11-11. Retrieved 2016-10-28.