Chapter 14: Storage and Shipping of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae
Printer friendly version [18 pages]
- Preservation and storage of isolates
It is often necessary for an isolate to be re-examined or further characterized at a time after the culture was initially obtained and tested. If isolates need to be sent to a national reference laboratory for confirmation of identity and/or further testing, they must be stored properly prior to packing and shipping. Selection of a storage method depends on the length of time the organisms are to be stored and the laboratory equipment and facilities available. Isolates to be prepared for either short-term or long-term storage should be confirmed as pure cultures before proceeding with any of these methodologies. Fresh cultures (i.e., 18-24 hour growth) should always be used for the preparation of isolates to be stored.
N. meningitidis, S. pneumoniae, and H. influenzae are fragile bacteria and care must be employed to preserve and transport them under the most ideal conditions possible. Aseptic techniques should be used at all times during the preparation of isolates for storage and/or transport to avoid contamination.
- Short-term storage
N. meningitidis, S. pneumoniae, and H. influenzae can only survive for 3-4 days on blood agar plates (BAP) and/or chocolate agar plates (CAP) and do not survive for long periods of time in broth; hence the need for effective and practical short-term and long-term term storage methods. Short-term storage methods are appropriate for bacterial isolates that only need to be stored for several days to a few weeks at a time. These methods include Dorset Transport medium, chocolate agar slants, and silica gel packages.
Dorset Transport medium
Dorset Transport medium can be used for room temperature (25°C) storage of N. meningitidis, S. pneumoniae, and H. influenzae (1). On Dorset Transport medium, N. meningitidis and H. influenzae can be stored for approximately 3 weeks, whereas S. pneumoniae can be stored for approximately 6 weeks. Instructions for preparation of Dorset Transport medium are included in the Annex. Dorset Transport medium is typically produced as a 4 ml slant in a 7 ml screw-cap tube. It should be stored at 4°C when not in use and warmed to room temperature (25°C) before use.
- Grow the pure isolate(s) to be stored for 18-24 hours on a BAP or a CAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Label the Dorset Transport slant with the Lab ID and the date.
- Use a disposable plastic loop to collect a few colonies of the overnight growth on the BAP or CAP and inoculate the Dorset Transport slant.
- Incubate overnight at 37°C in 5% CO2 then store the Dorset Transport slant at room temperature (25°C).
Chocolate agar slants
If Dorset Transport medium is not readily prepared or used by the laboratory, short-term storage of N. meningitidis, S. pneumoniae, and H. influenzae can be carried out on chocolate agar slants for up to 1 week. Instructions for preparation of chocolate agar slants are included in the Annex. Chocolate agar slants are typically produced as a 4 ml slant in a 7 ml screw-cap tube. They should be stored at 4°C when not in use and warmed to room temperature (25°C) before use.
- Grow the pure isolate(s) to be stored for 18-24 hours on a BAP or a CAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Label the chocolate agar slant with the Lab ID and the date.
- Use a disposable plastic loop to collect a few colonies of the overnight growth on the BAP or CAP and inoculate the chocolate agar slant.
- Incubate the slant for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar).
- Store the slant at room temperature (25°C).
- For N. meningitidis, solid screw-caps should be loosened during storage but permeable membrane screw caps (which allow for an exchange of gases and are commercially available) should be used when possible. An overlay of trypticase soy broth (TSB) may also be helpful and might increase viability. N. meningitidis slants should not be refrigerated.
- Viability is best for S. pneumoniae and H. influenzae if the slants are maintained at 4°C with the cap tightened to avoid drying after incubation.
Silica gel packages
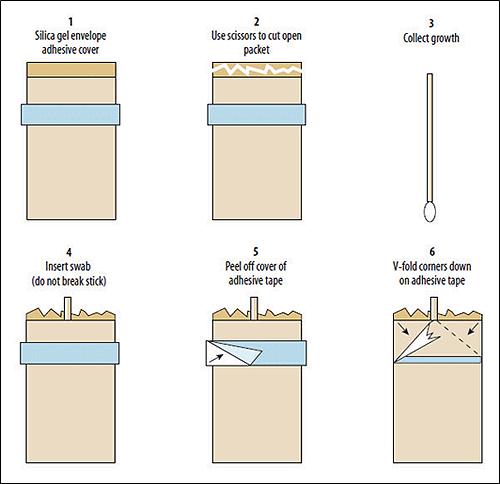
N. meningitidis, S. pneumoniae, and H. influenzae can also be stored short-term on swabs stored in silica gel packets, which are typically 1.5 g foil bags, with 75% white gel and 25% blue gel (the blue gel is added to detect moisture). Isolates can survive approximately 2 weeks at 4°C and perhaps slightly shorter at room temperature (25°C). The packets are inexpensive and easy to use (Figure 1), but are not often available from commercial manufacturers. Silica gel packages can be stored at room temperature (25°C) when not in use.
- Grow the pure isolate(s) to be stored for 18-24 hours on a BAP or a CAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Label the silica gel package with the Lab ID.
- Cut open the silica gel package near the adhesive cover with sterile scissors.
- Inspect the contents of the package. Blue and white gels should be visible. If only white gels are visible, that indicates that moisture was introduced into the silica gel package and the package should not be used.
- Collect the overnight pure culture growth from the BAP or CAP with a single sterile polyester swab.
- Do not use cotton swabs as cotton has a bacteriostatic effect which will inhibit bacteria growth.
- Place the swab in the silica gel package with the tip inserted into the silica and the shaft sticking out the top of the package.
- Remove the cover of adhesive tape on the silica gel package and fold down the corners to seal the package.
- Place additional tape around the shaft of the swab and folded corners to secure the swab and seal the package.
- Long-term storage
Long-term storage of bacterial isolates is best accomplished by either freezing or lyophilization (freeze-drying). Freezing is the most convenient storage method for frequently recovered isolates as the cultures do not need to thaw completely each time they are removed from frozen storage. Lyophilized bacteria can be stored for long periods at 4°C or -20°C and can be transported without refrigeration. However, the equipment required for this procedure is expensive and not all laboratories have the ability to lyophilize isolates. Reference laboratories choosing to lyophilize bacteria should always maintain a frozen preparation in addition to larger quantities of lyophilized strains as some lyophilized preparations may be nonviable upon reconstitution. Bacterial cultures may be stored frozen or lyophilized in a variety of storage media formulated for that purpose.
Frozen storage
Skim milk with glycerol, defibrinated sheep, horse, or rabbit blood, or Greaves medium is used for freezing. Instructions for preparing these media are listed in the Annex. Human blood should not be used due to safety issues (e.g., HIV and hepatitis transmission) and the possible inhibition of growth of isolates resulting from antibodies or residual antibiotics. Glass ampoules or vials for freezing in liquid nitrogen should not be used because they can explode upon removal from the freezer.
- Grow the pure isolate(s) to be stored for 18-24 hours on a BAP or a CAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Label the storage tube with the Lab ID and the date.
- Use a sterile Pasteur pipette to add 1 ml of well-mixed, sterile storage media to a 2 ml, externally-threaded, screw-cap cryovial.
- Use a sterile polyester-tipped swab to harvest all of the pure overnight growth from the BAP or CAP and inoculate the storage media by swirling the swab to release the organisms.
- Do not use cotton swabs as cotton has a bacteriostatic effect which will inhibit bacteria growth.
- Squeeze the excess media from the swab by rotating it against the sides of the cryovial before carefully withdrawing it. Discard the swab in disinfectant.
- If possible, rapidly freeze the suspension in a bath of 95% alcohol and dry ice pellets.
- Place the cryovials in a -70°C freezer or a liquid nitrogen freezer (-120°C). A -20°C freezer can be used, but some loss of viability can be expected.
- Freezers with automatic defrost cycles should never be used for the laboratory.
Lyophilization
Some laboratories may have lyophilization facilities. Serum-based media, skim milk, or polyvinylpyrrolidone (PVP) medium are generally used for lyophilization.
- Grow the pure isolate(s) to be stored for 18-24 hours on a BAP or a CAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Label the storage tube and/or lyophilization vial with the Lab ID and the date.
- Use a sterile Pasteur pipette to add 1-2 ml of well-mixed, sterile lyophilization medium to a 2 ml, externally-threaded, screw-cap cryovial.
- Use a sterile polyester-tipped swab to harvest all of the pure overnight growth from the BAP or CAP and suspend it into 1-2 ml of sterile lyophilization medium by swirling the swab to release the organisms.
- Do not use cotton swabs as cotton has a bacteriostatic effect which will inhibit bacteria growth.
- Squeeze the excess media from the swab by rotating it against the sides of the cryovial before carefully withdrawing it. Discard the swab in disinfectant.
- Place approximately 0.5 ml of the cell suspension into a sterile ampoule or lyophilization vial.
- Several vials can be prepared from a single plate, if desired.
- Sterility should be maintained at all times during preparation of the vial.
- Shell-freeze the cell suspension on the walls of the lyophilization vial. This is accomplished using one of the following two methods:
- Keep the lyophilization vial at -70°C until just before the cell suspension is added. Add the cell suspension and rapidly rotate the vial to freeze the suspension to the wall. Return the vial to the -70°C freezer until it is ready to be attached to the lyophilizer.
- Alternatively, if a -70°C freezer is not available, prepare a mixture of alcohol (95% ethanol) and dry ice. Add the cell suspension and rapidly rotate the lyophilization vial at a 45-60° angle in the alcohol and dry-ice mixture.
- Attach the vial to the lyophilizer and follow the manufacturer's instructions for lyophilization as the type of apparatus may vary slightly with each instrument.
- The time of lyophilization will depend on the number of vials being lyophilized and the capacity of the instrument. On an average machine, 4-5 hours are required to completely dry 10-20 small vials.
- Once the run is completed, seal the vials using a heat source or a capping mechanism while they are still attached to the lyophilizer and under a vacuum. The vials can be stored at 4°C or -20°C after being sealed.
- Recovery of N. meningitidis, S. pneumoniae, and H. influenzae isolates
Whether recovering an isolate from short-term or long-term storage, be sure to label all agar plates with the appropriate Lab ID and date using a permanent marker. Use the appropriate agar medium for each organism as specified in Chapter 6: Primary Culture and Presumptive Identification.
Dorset Transport medium and chocolate agar slants
- Use a sterile 10 µl loop to remove a loopful of growth from the Dorset Transport or chocolate agar slant and streak for isolation on a BAP or a CAP.
- Incubate the plate for 18–24 hours at 35-37°C with ~5% CO2 (or in a candle jar) and observe for growth
- If no growth is observed on the plate, repeat the above steps with another loopful of growth from the Dorset Transport or chocolate agar slant and streak for isolation on a BAP or a CAP.
Silica gel packages
- Remove the swab from the silica gel package and streak a vertical line down 1/3 of the center of a BAP or a CAP and then cross-streak 1/3 of the plate using the swab.
- Be sure that the entire surface area that contained the culture comes in contact with the plate.
- After streaking 1/3 of the plate, place the swab in approximately 1 ml of brain heart infusion (BHI) broth and incubate the broth for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar).
- Streak the remaining 2/3 of the plate for isolation with a 10 µl loop.
- Incubate the plate for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar) and observe for growth.
- If the organism is nonviable after overnight incubation, pipet 10 µl of the broth onto a new plate and streak for isolation. Incubate the plate for 18-24 hours at 35-37°C with ~5% CO2 and check for growth the next day.
Frozen isolates
- Allow frozen isolate to thaw at room temperature, just enough so that 10 µl of freezing medium can be removed from the top.
- Return frozen stock to the freezer immediately after collection. Once completely thawed, the frozen culture will begin to lose viability.
- Place 10 µl of freezing medium onto a BAP or a CAP and streak for isolation.
- Incubate the plate for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar) and observe for growth.
- If no growth is observed on the plate, repeat above steps with another 10 µl of the frozen culture.
- Isolates grown from frozen cultures should be subcultured at least once prior to being used in tests.
Lyophilized cultures
- Suspend lyophilized isolates in 0.25-0.5 ml of broth (e.g., TSB or Mueller-Hinton).
- Add 10 µl of the cell suspension to a BAP or a CAP and streak for isolation.
- Add approximately 50 ml of the suspension to a liquid broth containing 50 ml of blood (sheep, rabbit, goat, or horse blood, but not human blood).
- Incubate the plate for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar) and observe for growth.
- If growth of the appropriate bacteria is visible, the broth tube can be discarded.
- If no growth is observed on the plate, place 10 µl of the broth tube contents onto a new agar plate, streak for isolation, and incubate for 18-24 hours at 35-37°C with ~5% CO2 (or in a candle-jar).
- If the broth tube is not turbid, it is likely that the lyophilized sample is nonviable. This is why it is strongly suggested that a specimen be prepared for long-term frozen storage in addition to lyophilization.
- Isolates grown from lyophilized cultures should be subcultured at least once prior to being used in tests
- Short-term storage
- Preparation of infectious substances and diagnostic/patient specimens for packaging and shipping
Transport of diagnostic specimens and etiologic agents (infectious substances) should be done with care not only to minimize the hazard to humans and the environment, but also to protect the viability of the suspected pathogens. Transport of infectious material by public or commercial delivery systems may be subject to local, national, and international regulations.
If possible, specimens should be shipped so that they arrive in the receiving laboratory during working hours to ensure proper handling, prompt plating, and storage of the specimens. The receiving laboratory should be informed that the specimens are being shipped, preferably before the specimens are sent, so that appropriate arrangements can be made.
Depending on local conditions, within-country transport may be by ground or by air. If specimens are sent by a messenger, the messenger must know the location of the laboratory and the appropriate person to contact. The sender should identify a quick, inexpensive, and reliable mode of transport in advance and coordinate the shipping schedule and funding mechanism. For longer distances and international shipments, the quickest transport service may be air-freight or expedited delivery service. Because ice packs or dry ice for cold shipments will last only 24-48 hours, arrangements should be made for immediate collection at the receiving airport. In addition, the sender should coordinate with a contact at the destination to identify any special arrangements or criteria that need to be met for customs in that country. When specimens are shipped by air, the following information should be communicated immediately to the receiving laboratory: air freight company, air waybill number, flight number, times and dates of departure and arrival of the flight, and contents of the package.
- Preparation for transport of infectious specimens and cultures
N. meningitidis, S. pneumoniae, and H. influenzae specimens and isolates can be shipped frozen or on chocolate agar slants, in silica gel packages, or as lyophilized cultures. They should be packaged for transport as indicated below.
Frozen specimens and isolates
Frozen specimens and isolates should be shipped using a sufficient amount of ice packs or dry ice to maintain the proper cold temperature throughout the duration of the transportation, especially when shipping long distances or internationally. Ice packs will remain frozen for a day or two after the dry ice has dissipated. Glass vials should not be used when shipping frozen isolates. Guidelines for packaging infections substances and clinical specimens are listed in Sections IV and V, respectively.
Chocolate agar slants
Chocolate agar slants in screw-cap tubes should be shipped at room temperature (25°C). Isolates can survive for at least one week under these conditions.
Silica gel packages
Prepared silica gel packages should be shipped at 4°C (preferable) or at room temperature (25°C). Isolates can survive for up to 2 weeks in silica gel packages. Silica gel packages should be enclosed in 2 sealable bags within a plastic shipping container.
Lyophilized cultures
Lyophilized culture vials should be packaged according to the regulations specified in the WHO Laboratory Safety Manual, which is available at: http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/. Each vial or tube should be individually wrapped before being enclosed in a metal container along with enough absorbent material to absorb all liquid in the event of a spill. This container should then be enclosed in a cardboard shipping container and can be transported at room temperature (25°C). An address label and an etiological agent-warning label (EA label) should be attached to the shipping container. No more than 50 ml of culture can be shipped in one package.
- Preparation for transport of infectious specimens and cultures
- Regulations regarding transport and shipment of infectious substances and diagnostic/patient specimens
- Regulatory organizations
The United Nations Committee of Experts on the Transport of Dangerous Goods is continually developing recommendations for the safe transport of dangerous goods. The International Civil Aviation Organization (ICAO) has used these recommendations as the basis for developing regulations for the safe transportation of dangerous goods by air. The regulations of the International Air Transport Association (IATA) contain all the requirements of the ICAO Technical Instructions for the Safe Transport of Dangerous Goods. However, IATA has included additional requirements that are more restrictive than those of ICAO. Member airlines of the IATA have adopted the use of the IATA regulations governing dangerous goods and shippers must comply with these regulations in addition to any applicable regulations of the state of origin, transit, or destination.
The shipment of infectious substances or diagnostic specimens by air must comply with local, national, and international regulations. International air transport regulations may be found in the IATA publication titled Dangerous Goods Regulations. This reference is published annually in January and the regulations are updated each year. A copy of the IATA regulations in English, Spanish, French, or German may be obtained from one of the following regional offices.
Orders for IATA regulations from the Americas, Europe, Africa, and the Middle East:
Customer Service Representative
International Air Transport Association
800 Place Victoria, P.O. Box 113
Montreal, Quebec
CANADA H4Z 1M1
Telephone: +1 514 390 6726
Fax: +1 514 874 9659
Teletype: YMQTPXBOrders for IATA Regulations from Asia and the Pacific:
Customer Service Representative
International Air Transport Association
77 Robinson Rd.
No. 05-00 SIA Building
SINGAPORE 068896
Telephone: +65 438 4555
Fax: +65 438 4666
Telex: RS 24200 TMS Ref: TM 2883
Cable: IATAIATA
Teletype: SINPSXBInternet information:
www.iata.orgFor internet orders, send e-mail to:
sales@iata.org - Shipping regulations for infectious substances and diagnostic/patient specimens
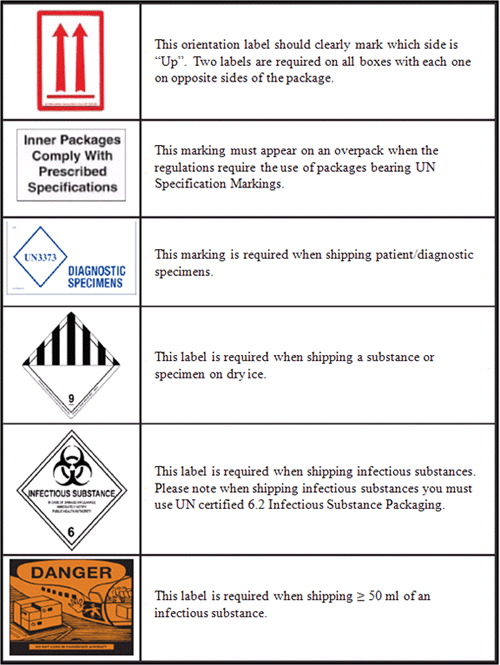
Packages that are shipped by air via commercial and cargo carriers (such as Federal Express, DHL, and passenger aircraft) are affected by IATA regulations. These regulations are outlined in this section of the laboratory manual to provide examples of acceptable packaging procedures for infectious materials. However, because they may not reflect current national or IATA requirements for packaging and labeling for infectious substances, anyone packaging isolates or infectious specimens should consult the appropriate national regulations and the current edition of the IATA Dangerous Goods Regulations before packing and shipping infectious substances by any means of transport (2). Table 1 includes images of labels and packages appropriate for shipping and different classifications of packages under IATA regulations. Note that a completed Shipper's Declaration for Dangerous Goods form is required for shipping hazardous materials including infectious substances. Instructions for completing this form are provided at the end of this section.
- Definition of infectious substances
According to IATA, infectious substances are defined as substances known or reasonably expected to contain pathogens. Pathogens are microorganisms (including bacteria, viruses, rickettsia, parasites, fungi) and other agents such as prions, which cause infectious disease in humans or animals.
- Definition of diagostic/patient specimens
According to IATA, diagnostic/patient specimens are those collected directly from humans or animals, including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention.
- Regulatory organizations
- Guidelines for packaging and labeling infectious substances
Persons who ship infectious agents must comply with all local, national, and international regulations pertaining to the packaging and handling of these materials. They must ensure that specimens arrive at their destination in good condition and that they present no hazard to humans or other organisms during transport.
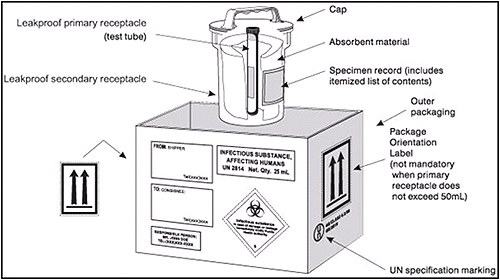
- The inner packaging of infectious substance shipments must include the following:
- An inner watertight primary container that is glass, metal, or plastic and has a leak-proof seal.
- Packaged infectious agents or diagnostic specimens which are placed in the primary container.
- Agar slants with screw-cap tops should be reinforced with adhesive tape. Petri plates should not be shipped.
- A watertight, impact-resistant secondary container
- United Nations (UN) Specification Packaging that has been rigorously tested and certified for infectious substances.
- The primary receptacle or the secondary packaging must be capable of withstanding, without leakage, an internal pressure producing a pressure differential of not less than 95 kPa and a temperature range of -40°C to 55°C (-40°F to 130°F).
- Absorbent material (such as cotton wool) between the primary container and the secondary container.
- If multiple primary containers are placed in a single secondary packaging, they must be wrapped individually, separated, and supported to ensure that contact between them is prevented. The absorbing material must be sufficient to absorb the entire contents of all primary containers.
- An itemized list of contents, placed between the secondary packaging and the outer packaging.
- The outer packaging of infectious substance shipments must meet the following requirements:
- Container should be of sufficient strength to adequately protect the contents.
- Size must be at least 100 mm (4 inches) in its smallest overall external dimension and of sufficient size to accommodate all labels to be placed on a single surface without overlapping.
- Package must be durably and legibly marked on the outside with the address and telephone number of the shipper and the consignee (the intended recipient).
- Infectious substance label must be affixed to the outside of the outer container and must bear the inscription, "Infectious substance. In case of damage or leakage, immediately notify public health authority."
- Marked with UN Specification Markings denoting that the packaging has been tested and certified for shipping infectious substances.
- Marked with the infectious substance marking (UN 2814): "Infectious substance, affecting humans (Genus species or technical name) x total number of milliliters or grams." The species can be specified or otherwise indicated as "spp." Note that this marking can be written by hand and does not require a special adhesive label. For example: "Infectious substance, affecting humans (N. meningitidis) x 5.0 ml" or "Infectious substance, affecting humans (Streptococcus spp.) x 5.0 ml" or "Infectious substance, affecting humans (HIV) x 0.5 ml".
- Labeled with a set of two up-arrows on at least two opposite sides of the outer box to indicate the proper package orientation for the closures to be in the upright position.
- Labeled on the top of the box with the statement "This End Up" or "This Side Up."
- Labeled with a "Cargo Aircraft Only" label if the total volume of the infectious substance per outer shipping container is ≥50 ml.
- Marked with the name and telephone number of the person responsible for the shipment.
The packaging requirements for transport of infectious substances are illustrated in Figure 2.
- The inner packaging of infectious substance shipments must include the following:
- Guidelines for packaging and labeling diagnostic/patient specimens
Diagnostic/patient (clinical) specimens with a low probability of containing an infectious agent must be packaged as follows, so that will not leak after a 1.2 meter drop test procedure:
- Contents should be triple packed with a watertight primary container, a leak-proof secondary container, and sufficient absorbent material to absorb all liquid in case of a spill packed in between the primary and secondary containers.
- The primary receptacle or the secondary packaging must be capable of withstanding, without leakage, an internal pressure differential of 95 KiloPascals between -40°C and 55°C. Infectious substance containers exceed these criteria and are therefore acceptable for use for packing and shipping of diagnostic specimens.
- Include an itemized list of contents between the secondary packaging and the outer packaging.
- Marked with the "diagnostic specimens" statement on the outside of the outer container: "Diagnostic specimen. UN 3373. Packed in compliance with IATA Packing Instruction 650." This marking can be written by hand and does not require a special adhesive label.
- If being shipped by air, the diagnostic specimens statement "Diagnostic specimen. UN 3373. Packed in compliance with IATA Packing Instruction 650" must be present on the air waybill as well as on the outer container. The packaging requirements for transport of diagnostic specimens are illustrated in Figure 3.
- Guidelines for packaging and labeling of specimens shipped on dry ice (CO2)
- Dry ice must be placed outside the secondary packaging in an overpack and interior supports must be provided to secure the secondary packaging in the original position after the ice has dissipated.
- Dry ice must be packed according to IATA Packing Instruction 954: the outer packaging must permit the release of carbon dioxide (CO2) gas. Cardboard and polystyrene foam are two examples of materials suitable for the packaging of dry ice. In a temperate climate, approximately 6 pounds of dry ice will dissipate in a 24 hour period and are therefore suitable for a 24 hour shipment. This amount should be adjusted accordingly for warmer climates and size of the box to ensure that the contents remain frozen. For air transport, the maximum dry ice allowed in a single outer container is 200 kg (approximately 440 pounds).
- Packages containing dry ice must be properly marked with the statement "Carbon dioxide, solid (dry ice); UN1845; (and net weight of the dry ice in kg)," and a preprinted Class 9 "Miscellaneous Dangerous Goods" label, as shown in Table 1.
- When an overpack is used, it must be marked with the statement "Inner packages comply with prescribed specifications" because the UN Specification Markings will not be visible on the outer-most packaging.
- Guidelines for completion of the "Shipper's Declaration for Dangerous Goods" form
All shipments of hazardous materials, including infectious substances, must be accompanied by two original completed copies of the "Shipper's Declaration for Dangerous Goods" form, along with the other shipping documents. It is important to remember the following in order to reduce the risk of a shipment being refused and returned to the laboratory of origin:
- International regulations require the diagonal hatch marks in the left and right margins to be printed in red. Therefore, black and white photocopies of this form may not be used.
- The form must be completed in English, although translations may accompany it on the same form.
- Specific terms, spellings, and nomenclature must be used. For example, a cardboard box must be referred to as "fibreboard box" (spelled with R before E), and there must be a comma after the term "infectious substance" within the statement "infectious substance, affecting humans".
- The person responsible for the shipment must be listed in one of the address boxes. If the person responsible for the shipment is different than the shipper or recipient, the responsible person's telephone number should be included alongside the name.
- Under the "Transport Details" portion of the form, cross out the option that does not apply. If the shipment is less than 50 ml, cross out "cargo aircraft only." If the shipment is greater than 50 ml, cross out "passenger and cargo aircraft."
- Under the "Nature and Quantity of Dangerous Goods" portion of the form, the proper shipping name for infectious substances is "Infectious substance, affecting humans (technical name)." The technical name of the infectious substance(s) must be included in parentheses after the proper shipping name; however, the specific species is not required and "spp." may follow the genus. The technical name of the infectious substance Neisseria meningitidis should be listed as either "(Neisseria meningitidis)" or "(Neisseria spp.)."
- For "Infectious substances, affecting humans (technical name)": the proper class is Division 6.2, the UN number is UN2814, and the packing instruction is 620.
- For "Carbon dioxide, solid (dry ice)": the proper class is Class 9 Miscellaneous Dangerous Good, the UN number is UN1845, the packing group is III, and the packing instruction is 954.
- For infectious substances, the quantity must be noted in ml under the "Quantity and Type of Packing" portion of the form.
- For dry ice, the quantity must be noted in kg (measured in whole numbers) under the "Quantity and Type of Packing" portion of the form.
- If the UN specification marking is not visible on the outer package, the declaration must contain the statement "OVERPACK USED" under the "Quantity and Type of Packing" portion of the form.
- Under the "Additional Handling Information" portion of the form, the 24 hour emergency contact telephone number must be answered by a person knowledgeable about emergency response procedures for damaged and leaking boxes.
- The "Shipper's Declaration for Dangerous Goods" form is a legal document and must be signed.
It is important to communicate shipping details to the intended recipient prior to shipment of the package. In addition, arrangements should be made for proper handling during shipping and legal importation of the infectious substance to ensure delivery without delay. These guidelines are in accordance with IATA regulation 1.3.3.1.
References
- Wasas, A.D., Huebner, R.E., and Klugman, K. 1999. Use of Dorset Egg Medium for Maintenance and Transport of Neisseria meningitidis and Haemophilus influenzae Type b. Journal of Clinical Microbiology. 37:2045–6.
- International Air Transport Association Dangerous Goods Regulations 52nd Edition, Effective January 1, 2011. Montreal.
- Page last reviewed: April 15, 2016
- Page last updated: March 15, 2012
- Content source:


 ShareCompartir
ShareCompartir