Cardiopulmonary bypass
Cardiopulmonary bypass (CPB) is a technique in which a machine temporarily takes over the function of the heart and lungs during surgery, maintaining the circulation of blood and the oxygen content of the patient's body. The CPB pump itself is often referred to as a heart–lung machine or "the pump". Cardiopulmonary bypass pumps are operated by perfusionists. CPB is a form of extracorporeal circulation. Extracorporeal membrane oxygenation is generally used for longer-term treatment.
| Cardiopulmonary bypass | |
|---|---|
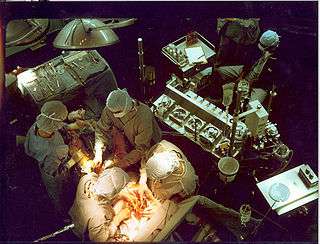 A heart–lung machine (upper right) in a coronary artery bypass surgery. | |
| ICD-9-CM | 39.61 |
| MeSH | D002318 |
| OPS-301 code | 14 |
| Other codes | 22570829 |
Uses
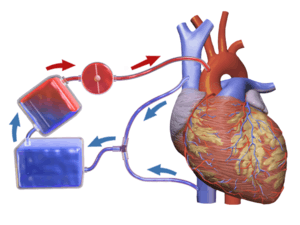
Cardiopulmonary bypass is commonly used in coronary bypass heart surgery because of the difficulty of operating on the beating heart. Operations requiring the opening of the chambers of the heart requires the use of CPB to avoid engulfing air systemically and to provide a bloodless field to increase visibility for the surgeon. The machine pumps the blood and, using an oxygenator, allows red blood cells to pick up oxygen, as well as allowing carbon dioxide levels to decrease. This mimics the function of the heart and the lungs, respectively.
CPB can be used for the induction of total body hypothermia, a state in which the body can be maintained for up to 45 minutes without perfusion (blood flow). If blood flow is stopped at normal body temperature, permanent brain damage normally occurs in three to four minutes – death may follow shortly afterward. Similarly, CPB can be used to rewarm individuals suffering from hypothermia.[1] This rewarming method of using CPB is successful if the core temperature of the patient is above 16 °C. [2]
Extracorporeal membrane oxygenation (ECMO) is a simplified version of the heart lung machine that includes a centrifugal pump and an oxygenator to temporarily take over the function of heart and/or the lungs. ECMO is useful in post cardiac surgery patients with cardiac or pulmonary dysfunction, in patients with acute pulmonary failure, massive pulmonary embolisms, lung trauma from infections, and a range of other problems that impair cardiac or pulmonary function. ECMO gives the heart and/or lungs time to repair or recover but it's only a temporary solution. Patients with terminal conditions, cancer, severe nervous system damage, uncontrolled sepsis and other conditions may not be candidates for ECMO.[3]
CPB mechanically circulates and oxygenates blood for the body while bypassing the heart and lungs. It uses a heart–lung machine to maintain perfusion to other body organs and tissues while the surgeon works in a bloodless surgical field. The surgeon places a cannula in the right atrium, vena cava, or femoral vein to withdraw blood from the body. The cannula is connected to tubing filled with isotonic crystalloid solution. Venous blood which is removed from the body by the cannula is filtered, cooled or warmed, oxygenated, and then returned to the body. The cannula used to return oxygenated blood is usually inserted in the ascending aorta, but it may be inserted in the femoral artery. The patient is administered heparin to prevent clotting, and protamine sulfate is given after to reverse effects of heparin. During the procedure, hypothermia may be maintained; body temperature is usually kept at 28 °C to 32 °C (82.4–89.6 °F). The blood is cooled during CPB and returned to the body. The cooled blood slows the body's basal metabolic rate, decreasing its demand for oxygen. Cooled blood usually has a higher viscosity, but the crystalloid solution used to prime the bypass tubing dilutes the blood.
Surgical procedures in which cardiopulmonary bypass is used
- Coronary artery bypass surgery
- Cardiac valve repair and/or replacement (aortic valve, mitral valve, tricuspid valve, pulmonic valve)
- Repair of large septal defects (atrial septal defect, ventricular septal defect, atrioventricular septal defect)
- Repair and/or palliation of congenital heart defects (Tetralogy of Fallot, transposition of the great vessels)
- Transplantation (heart transplantation, lung transplantation, heart–lung transplantation, liver transplantation)
- Repair of some large aneurysms (aortic aneurysms, cerebral aneurysms)
- Pulmonary thromboendarterectomy
- Pulmonary thrombectomy
- Isolated Limb perfusion[4]
History

An Austrian-German physiologist Maximilian von Frey constructed an early prototype of a heart-lung machine in 1885 at Carl Ludwig’s Physiological Institute of the University of Leipzig.[5] However, such machines were not feasible before the discovery of heparin in 1916 which prevents blood coagulation. A Soviet scientist Sergei Brukhonenko developed a heart-lung machine for total body perfusion in 1926 which was used in experiments with canines. A team of scientists at the University of Birmingham (including Eric Charles, a chemical engineer) were among the pioneers of this technology.[6][7]
Dr. Clarence Dennis led the team at the University of Minnesota Medical Center that on April 5, 1951, conducted the first human operation involving open cardiotomy with temporary mechanical takeover of both heart and lung functions. The patient did not survive due to an unexpected complex congenital heart defect. One member of the team was Dr. Russell M. Nelson, who later became president of The Church of Jesus Christ of Latter-day Saints, and who performed the first open heart surgery in Utah.[8]
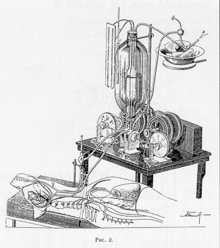
The first successful mechanical support of left ventricular function was performed in July 3, 1952 by Forest Dewey Dodrill using a machine, the Dodrill-GMR co-developed with General Motors. The machine was later used to support right ventricular function.[9]
The first successful open heart procedure on a human utilizing the heart lung machine was performed by John Gibbon and Frank F. Allbritten, Jr.[10] on May 6, 1953 at Thomas Jefferson University Hospital in Philadelphia. They repaired an atrial septal defect in an 18-year-old woman.[11] Gibbon's machine was further developed into a reliable instrument by a surgical team led by John W. Kirklin at the Mayo Clinic in Rochester, Minnesota in the mid-1950s.[12]
The oxygenator was first conceptualized in the 17th century by Robert Hooke and developed into practical extracorporeal oxygenators by French and German experimental physiologists in the 19th century. Bubble oxygenators have no intervening barrier between blood and oxygen, these are called 'direct contact' oxygenators. Membrane oxygenators introduce a gas-permeable membrane between blood and oxygen that decreases the blood trauma of direct-contact oxygenators. Much work since the 1960s focused on overcoming the gas exchange handicap of the membrane barrier, leading to the development of high-performance microporous hollow-fibre oxygenators that eventually replaced direct-contact oxygenators in cardiac theatres.[13]
In 1983, Ken Litzie patented a closed emergency heart bypass system which reduced circuit and component complexity.[14] This device improved patient survival after cardiac arrest because it could be rapidly deployed in non-surgical settings.[15]
Components
Cardiopulmonary bypass consists of two main functional units, the pump and the oxygenator which removes relatively oxygen-depleted blood from a patient's body and replaces it with oxygen-rich blood through a series of tubes (hoses).
Tubing
The components of the CPB circuit are interconnected by a series of tubes made of silicone rubber or PVC.[16]
Pumps
Roller pump
The pump console usually comprises several rotating motor-driven pumps that peristaltically "massage" tubing. This action gently propels the blood through the tubing. This is commonly referred to as a roller pump, or peristaltic pump.
Centrifugal pump
Many CPB circuits now employ a centrifugal pump for the maintenance and control of blood flow during CPB. By altering the speed of revolution (RPM) of the pump head, blood flow is produced by centrifugal force. This type of pumping action is considered to be superior to the action of the roller pump by many because it is thought to produce less blood damage (hemolysis, etc.), but mostly due to the centrifugal pump being pressure limited, therefore less likely to rupture the system if a sudden occlusion occurs on the high-pressure line system.
Oxygenator
The oxygenator is designed to add oxygen to infused blood and remove some of the carbon dioxide from the venous blood. Cardiac surgery was made possible by CPB using bubble oxygenators, but membrane oxygenators have supplanted bubble oxygenators since the 1980s. The main reasons for this are that membrane oxygenators tend to generate many fewer micro-bubbles, referred to as gaseous microemboli, which is generally considered harmful to the patient [17] and reduce damage to blood cells,[18] compared to bubble oxygenators.
Another type of oxygenator gaining favour recently is the heparin-coated blood oxygenator which is believed to produce less systemic inflammation and decrease the propensity for blood to clot in the CPB circuit.
Cannulae
Multiple cannulae are sewn into the patient's body in a variety of locations, depending on the type of surgery. A venous cannula removes oxygen depleted venous blood from a patient's body. An arterial cannula infuses oxygen-rich blood into the arterial system. A cardioplegia cannula delivers a cardioplegia solution to cause the heart to stop beating.
Some commonly used cannulation sites:
| Venous | Arterial | Cardioplegia |
|---|---|---|
| Right atrium | Proximal aorta, distal to the cross-clamp | Proximal aorta, proximal to the cross-clamp |
| Vena cavae | Femoral artery | Coronary sinus (retrograde delivery) |
| Femoral vein | Axillary artery | Coronary ostia |
| Distal aorta | Bypass grafts (during CABG) | |
| Apex of the heart | ||
Cardioplegia
A CPB circuit consists of a systemic circuit for oxygenating blood and reinfusing blood into a patient's body (bypassing the heart); and a separate circuit for infusing a solution into the heart itself to produce cardioplegia (i.e. to stop the heart from beating), therefore providing myocardial protection (i.e. to prevent the death of heart tissue).
Operation
A CPB circuit must be primed with fluid and all air expunged from the arterial line/cannula before connection to the patient. The circuit is primed with a crystalloid solution and sometimes blood products are also added. The patient must be fully anticoagulated with an anticoagulant such as heparin to prevent massive clotting of blood in the circuit.
Complications
CPB is not benign and there are a number of associated problems:
- Postperfusion syndrome (also known as "pumphead")
- Hemolysis
- Capillary leak syndrome
- Clotting of blood in the circuit – can block the circuit (particularly the oxygenator) or send a clot into the patient.
- Air embolism
- Leakage – a patient can rapidly exsanguinate (lose blood perfusion of tissues) if a line becomes disconnected.
- 1.5% of patients that undergo CPB are at risk of developing Acute Respiratory Distress Syndrome.
As a consequence, CPB is only used during the several hours a cardiac surgery may take. Most oxygenators come with a manufacturer's recommendation that they are only used for a maximum of 6 hours, although they are sometimes used for up to 10 hours, with care being taken to ensure they do not clot off and stop working. For longer periods than this, an ECMO (extracorporeal membrane oxygenation) is used, which can be in operation for up to 31 days – such as in this Taiwanese case, for 16 days, after which the patient received a heart transplant.[19]
CPB may contribute to immediate cognitive decline. The heart-lung blood circulation system and the connection surgery itself release a variety of debris into the bloodstream, including bits of blood cells, tubing, and plaque. For example, when surgeons clamp and connect the aorta to tubing, resulting emboli may block blood flow and cause mini strokes. Other heart surgery factors related to mental damage may be events of hypoxia, high or low body temperature, abnormal blood pressure, irregular heart rhythms, and fever after surgery.[20]
References
- McCullough, L.; Arora, S. (Dec 2004). "Diagnosis and treatment of hypothermia". Am Fam Physician. 70 (12): 2325–32. PMID 15617296.
- Lich, Bryan; Brown, Mark (2004). The Manual of Clinical Perfusion (2nd ed.). Fort Myers, Florida: PERFUSION.COM, INC. p. 117. ISBN 978-0-9753396-0-2.
- Lich, Bryan (2004). Manual of Clinical Pefusion (2nd ed.). Fort Myers, Florida: perfusion.com. p. 141. ISBN 978-0-9753396-0-2.
- Lich, Bryan (2004). The Manual of Clincal Perfusion (2nd ed.). Fort myers, Florida: Perfusion.com. p. 117. ISBN 978-0-9753396-0-2.
- Zimmer, Heinz-Gerd (September 2003). "The heart-lung machine was invented twice--the first time by Max von Frey". Clinical Cardiology. 26 (9): 443–5. doi:10.1002/clc.4960260914. ISSN 0160-9289. PMC 6654655. PMID 14524605.
- Dennis C; Spreng DS; Nelson GE; et al. (October 1951). "Development of a Pump-oxygenator to Replace the Heart and Lungs: An Apparatus Applicable to Human Patients and Application to One Case". Ann. Surg. 134 (4): 709–21. doi:10.1097/00000658-195110000-00017. PMC 1802968. PMID 14878382.
- Corporation, Bonnier (1 February 1951). "Popular Science". Bonnier Corporation. Retrieved 4 April 2018 – via Google Books.
- "U of U Health - Celebrating 60 Years of Cardiac Surgery in Utah With Russell M. Nelson, M.D." utah.edu. Archived from the original on 17 January 2018. Retrieved 4 April 2018.
- Norton, Jeffrey (2008). Surgery: Basic science and clinical evidence. NY: springer. p. 1473. ISBN 978-0-387-30800-5.
- Hedlund, Kelly D. A Tribute to Frank F. Allbritten, Jr. Origin of the Left Ventricular Vent during the Early Years of Open-Heart Surgery with the Gibbon Heart-Lung Machine. Texas Heart Institute Journal, Tex Heart Inst J. 2001; 28(4): 292–296. Summer 2001. Retrieved May 18, 2019.
- Cohn LH (May 2003). "Fifty years of open-heart surgery". Circulation. 107 (17): 2168–70. doi:10.1161/01.CIR.0000071746.50876.E2. PMID 12732590.
- "John Kirklin Cardiac Surgery Pioneer Dead at Age 86." (April 23, 2004) University of Alabama at Birmingham. press release
- Lim M (2006). "The history of extracorporeal oxygenators". Anaesthesia. 61 (10): 984–95. doi:10.1111/j.1365-2044.2006.04781.x. PMID 16978315.
- "US Patent for Emergency bypass system Patent (Patent # 4,540,399 issued September 10, 1985) - Justia Patents Search". patents.justia.com. Retrieved 2019-09-28.
- Reichman, Robert (1990). "Improved Patient Survival Using a Cardiopulmonary Support System After Cardiac Arrest". Annals of Thoracic Surgery. 49: 101–105.
- Davies, Huw. "Cardiopulmonary bypass machine - CPB". www.ebme.co.uk. Retrieved 2019-11-21.
- Pearson, D.T.; Holden M; Poslad S; Murray A; Waterhouse P. (1984). "A clinical comparison of the gas transfer characteristics and gaseous microemboli production of one membrane and five bubble oxygenators: gas transfer characteristics and gaseous microemboli production". Perfusion. 1 (1): 15–26. doi:10.1177/026765918600100103.
- Pearson, D.T.; Holden M; Poslad S; Murray A; Waterhouse P. (1984). "A clinical comparison of the gas transfer characteristics and gaseous microemboli production of one membrane and five bubble oxygenators: haemocompatibility". Perfusion. 1 (1): 81–98. doi:10.1177/026765918600100103.
- Man survives 16 days without a heart united Press International. April 3, 2008.
- Stutz, Bruce "Pumphead: Does the heart-lung machine have a dark side?" Scientific American, January 9, 2009.
External links
| Wikimedia Commons has media related to Cardiopulmonary bypass. |
- International Consortium For Evidence-Based Perfusion
- CircuitSurfers: A Perfusion Blog about Cardiopulmonary Bypass
- Hessel EA, Edmunds LH (2003). "Extracorporeal Circulation: Perfusion Systems". In Cohn LH, Edmunds LH (eds.). Cardiac Surgery in the Adult. New York: McGraw-Hill. pp. 317–38. Archived from the original on 2006-12-10. Retrieved 2006-12-09.
- Multimedia Manual of Cardiothoracic Surgery. Cardiopulmonary bypass collection.
- Profiles in Science: The Clarence Dennis Papers Selected papers of Clarence Dennis, credited with the first attempt at cardiopulmonary bypass surgery.