Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) Q&A;
 Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE)
Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE)
The College of Medicine and Allied Health Sciences (COMAHS), University of Sierra Leone, the Sierra Leone Ministry of Health and Sanitation (MoHS), and the U.S. Centers for Disease Control and Prevention (CDC) are working together on an Ebola vaccine clinical trial in Sierra Leone.
What is STRIVE?
STRIVE stands for the Sierra Leone Trial to Introduce a Vaccine against Ebola. It is a combined Phase 2 and Phase 3 clinical trial designed to assess the safety and efficacy of the rVSV-ZEBOV candidate Ebola vaccine. STRIVE is expanding on the information on safety and immune response available from previous smaller studies. Learn more about clinical trial phases.
Where is STRIVE taking place?
The study is being conducted in Sierra Leone, one of the countries heavily affected by the 2014-2015 Ebola outbreak. The study is taking place in five districts in Sierra Leone:
- Western Area Rural
- Western Area Urban, which contains Freetown
- Port Loko (3 chiefdoms)
- Bombali (3 chiefdoms)
- Tonkolili (1 chiefdom)
These study locations were selected because they were heavily affected by Ebola. The selection was also based on several logistical considerations, such as the ability to store the vaccine and sufficient space to enroll and vaccinate study participants.
Who has participated in STRIVE?
STRIVE has enrolled more than 8,650 health and other frontline workers, like:
- Doctors and nurses
- Cleaning, laboratory, pharmacy, security, and administrative health facility staff
- Ambulance teams
- Workers responsible for swabbing deceased people
- Surveillance teams
- Burial workers
Participants were eligible to enroll if they worked in the districts and chiefdoms selected for the study and were 18 years of age or older. Pregnant women, breastfeeding women, and people with weakened immune systems were not enrolled this study. Taking part in the study is voluntary.
What is the study design for STRIVE?
Unblinded: STRIVE researchers and each participant will know if he or she has gotten the vaccine.
Individually Randomized: A process is used to assign each STRIVE participant by chance to either get the vaccine in the immediate or the deferred vaccination group.
This study is an unblinded, individually randomized trial with phased introduction of the vaccine. A placebo was not used. Enrolled participants were randomized to either the immediate or deferred vaccination group. Participants in the immediate group were vaccinated within a week of enrollment and participants in the deferred group were vaccinated later in the study (about six months after enrollment).
Study staff will monitor participants’ health from the time they enroll until six months after getting vaccinated.
The first 400 study participants enrolled in STRIVE participated in a sub-study that looked frequently and in detail at adverse events following vaccination (this is known as reactogenicity). Adverse events included such things as fever, redness at the injection site, or headache, which may or may not be related to the vaccine. Half of these participants were randomized to get the vaccine immediately, and the other half about six months later. In addition to having usual study procedures with follow up phone calls monthly from the time they enrolled until six months after getting vaccinated, study staff followed up with these participants on specific days during the first month after enrollment and asked about specific symptoms. These participants were also asked to keep a diary to help them remember any symptoms they had.
An immunogenicity sub-study is being conducted among approximately 500 STRIVE participants. Immunogenicity is the ability of the vaccine to produce an immune response, or stimulate the body to develop antibodies. These study participants had their blood drawn before vaccination and will have it drawn three additional times over the course of the year to test antibody levels.
What is known about the vaccine used in STRIVE?
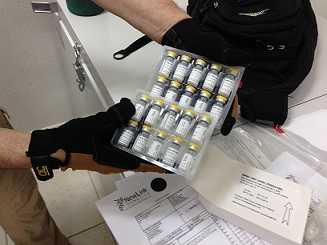
Ebola candidate vaccine is readied for frontline worker study in Sierra Leone.
The vaccine used in STRIVE is rVSV-ZEBOV, or recombinant Vesicular Stomatitis Virus Zaire ebolavirus vaccine. To make this vaccine, the vesicular stomatitis virus is weakened by removing one of its genes. That gene is replaced with a single Ebola virus gene. The vaccine is designed to stimulate the person’s immune system to produce antibodies against Ebola. The vaccine cannot cause Ebola because it does not contain the whole Ebola virus. The vaccine only contains a single gene of the Ebola virus. Only the whole Ebola virus can cause Ebola.
This vaccine was designed to help protect against Zaire ebolavirus, the virus that is causing the current outbreak in West Africa.
Other studies of the rVSV-ZEBOV candidate Ebola vaccine have been conducted and continue in Canada, Gabon, Germany, Kenya, Switzerland, and the United States. These studies suggest that the vaccine may help protect people from getting Ebola, but it is not yet known how much protection the vaccine may provide. To know this, the vaccine needs to be studied in more people and in places affected by Ebola. In addition to in Sierra Leone, this vaccine is also being used in Phase 2 and Phase 3 trials in Guinea and Liberia.
Researchers at the Public Health Agency of Canada’s National Microbiology Laboratory developed the candidate Ebola vaccine and licensed it to a subsidiary of NewLink Genetics Corp. NewLink entered into a licensing and collaboration agreement with Merck to research, develop, manufacture, and distribute the vaccine.
What are the possible side effects of the vaccine used in STRIVE?
Information about side effects is available from Phase 1 studies. As with any vaccine, this one can cause mild side effects. Common side effects with this vaccine include sore arm, making someone tired, or giving them a fever, headache, or muscle ache. One out of two people who get vaccinated may experience one or more of these reactions. Others, about 1 out of 8 people, may feel nauseous. These side effects usually happen within the first 24 hours after getting vaccinated and typically get better in 1 to 2 days.
Some people who get this vaccine might get mildly painful swelling of the joints. In one study where this side effect was reported, it occurred in about 1 out of 7 people who got the vaccine. In those who have this side effect, it typically appears in the second week after getting vaccinated and usually gets better in about 1 to 2 weeks or less. Other people may have a mild skin rash, painless blisters on the hands or feet, or mouth ulcers. In one study where these side effects were reported, they occurred in about 1 out of 20 people who got the vaccine. They typically appear in the second week after vaccination and usually get better in about 1 to 2 weeks or less.
Like with other candidate vaccine studies, when larger numbers of people get vaccinated, additional side effects may be seen.
How is the safety of STRIVE participants being addressed?
The well-being and safety of study participants is an important priority. The STRIVE protocol was reviewed and approved by the Sierra Leone Ethics and Scientific Review Committee, the Sierra Leone Pharmacy Board, and the CDC Institutional Review Board. Also, the U.S. Food and Drug Administration said the study can proceed. These reviews ensured that the study would be scientifically, ethically, and clinically appropriate and that the study adheres to internationally accepted standards for protecting research participants.
Volunteers who meet the study’s eligibility criteria must provide written informed consent to participate. Throughout the course of the trial, an independent Data and Safety Monitoring Board comprised of clinical research experts, statisticians, ethicists, and community representatives have been meeting regularly to review data as it is collected.
If a study participant develops a sudden illness, severe symptoms due to any cause, or gets Ebola during their participation in STRIVE, he or she will be provided with free medical care at a designated health facility in Sierra Leone.
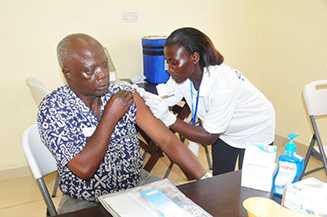
Vaccination in Western Urban district.
Can STRIVE participants still get Ebola after getting the vaccine?
This is possible, because it is not yet known if the vaccine will provide protection or how much protection it may provide. If the vaccine does offer protection against Ebola, it could take a few weeks for the body to develop any protection. STRIVE participants were instructed to follow recommended measures to prevent against exposure to the Ebola virus; this may include use of personal protective equipment (PPE) and other infection control practices.
Were STRIVE participants compensated?
Yes, participants received some reimbursement to help with their costs of time away from work and transportation.
How will the decrease in new Ebola cases and the declaration in November, 2015, that the Ebola epidemic is over in Sierra Leone affect STRIVE?
Study partners and staff congratulate Sierra Leone on ending the Ebola epidemic. Researchers understand that because cases were declining when STRIVE started and because there are no cases in Sierra Leone currently, STRIVE will not be able to determine vaccine efficacy, or definitively conclude how well the vaccine works. However, even though efficacy cannot be determined, STRIVE will expand the safety information available for the rVSV-ZEBOV candidate vaccine, likely creating the largest safety database available on the vaccine, and provide immunogenicity and safety data that may contribute to vaccine licensure.
What impact will STRIVE have in Sierra Leone beyond the trial?
The study is strengthening existing research capacity of institutions in Sierra Leone by providing training and research experience to hundreds of Sierra Leonean staff. The skills and expertise of these trained personnel can be used in future studies. Infrastructure has been expanded, including renovating existing structures and building new ones to be able to enroll and vaccinate participants, handle data management, and store the vaccine. New technology is also available to maintain the cold chain, which keeps vaccines at required temperatures.
Related Links
- Page last reviewed: April 20, 2016
- Page last updated: April 20, 2016
- Content source:




 ShareCompartir
ShareCompartir