Latent Tuberculosis Infection: A Guide for Primary Health Care Providers
Diagnosis of Latent TB Infection
The diagnosis of LTBI is based on information gathered from the medical history, TST or IGRA result, chest radiograph, physical examination, and in certain circumstances, sputum examinations. The presence of TB disease must be excluded before treatment for LTBI is initiated because failure to do so may result in inadequate treatment and development of drug resistance (see Table 1).
CDC discourages use of diagnostic tests for LTBI among individuals and populations at low risk for infection with M. tuberculosis. Despite CDC recommendations to the contrary, testing is sometimes done to meet administrative or legal requirements for groups who are not considered to have an increased possibility of infection in the absence of other factors cited above, such as persons meeting entrance requirements for certain schools and workplaces.
Table 1:
Differentiating Between Latent TB Infection and TB Disease
-
LTBI
- No symptoms or physical findings suggestive of TB disease.
- TST or IGRA result usually positive.
- Chest radiograph is typically normal.
- If done, respiratory specimens are smear and culture negative.
- Cannot spread TB bacteria to others.
- Should consider treatment for LTBI to prevent TB disease.
-
TB Disease
- Symptoms may include one or more of the following: fever, cough, chest pain, weight loss, night sweats, hemoptysis, fatigue, and decreased appetite.
- TST or IGRA result usually positive.
- Chest radiograph is usually abnormal. However, may be normal in persons with advanced immunosuppression or extrapulmonary disease.
- Respiratory specimens are usually smear or culture positive. However, may be negative in persons with extrapulmonary disease or minimal or early pulmonary disease.
- May spread TB bacteria to others.
- Needs treatment for TB disease.
Tests for TB Infection
Tuberculin Skin Test (TST)
The TST is used to determine if a person is infected with M. tuberculosis. If a person is infected, a delayed-type hypersensitivity reaction is detectable 2 to 8 weeks after infection. The skin test is administered intradermally using the Mantoux technique by injecting 0.1ml of 5 TU purified protein derivative (PPD) solution. The reading and interpretation of TST reactions should be conducted within 48 to 72 hours of administration. For more information about tuberculin skin testing, visit the CDC website for additional resources (see Resources) and refer to Appendix C.
Key Points
- Training is essential for health care providers to gain proficiency in the administration and interpretation of the TST.
- The TST should not be performed on a person who has written documentation of either a previous positive TST result or treatment for TB disease.
- Patients or family members should never measure TST results; this should only be done by a trained health care professional.
- Interpretation of the TST result is the same for persons who have had BCG vaccination because a majority of BCG cross-reactivity wanes with time.
- A TST that was not measured and recorded in millimeters (mm) of induration must be repeated.
Classification of Tuberculin Skin Test Reactions
Interpretation of TST results is based on the measurement of the reaction in millimeters, the person’s risk of acquiring TB infection, or the risk of progression to disease if infected. See the risk stratification below.
Generic Textbox module not found Generic Textbox module not foundGeneric Textbox module not found
Although skin testing activities should be conducted only among at-risk groups, certain individuals may be required to have testing for employment or school attendance independent of risk. CDC and ATS do not recommend a testing approach that is independent of a risk assessment.
Interferon–Gamma Release Assays (IGRAs)
IGRAs are used to determine if a person is infected with M. tuberculosis by measuring the immune response to TB proteins in whole blood. Specimens are mixed with peptides that simulate antigens derived from M. tuberculosis and controls. In a person infected with
M. tuberculosis, the white blood cells recognize the simulated antigens and release interferon-gamma (IFN- γ); results are based on the amount of IFN- γ released.
As noted earlier, there are 2 U.S. Food and Drug Administration (FDA) approved IGRAs commercially available in the United States:
- QuantiFERON®-TB Gold-in-Tube test (QFT-GIT)
- T-SPOT® TB test
Key Points
- Advantages of IGRAs include the following:
- Requires a single patient visit to conduct the test.
- Does not cause booster phenomenon.
- Laboratory test not affected by health care worker perception
or bias. - Results can be available within 24 hours.
- Unaffected by BCG and most environmental mycobacteria.
- Limitations of IGRAs include the following:
- Blood sample must be processed within 8-30 hours after collection.
- Limited data exist on use in groups such as children younger than 5 years of age, persons recently exposed to TB, immunocompromised persons, and those who will be tested repeatedly (serial testing).
Interpretation of IGRA Results
The interpretation of IGRAs is based on the amount of IFN-γ released, in QFT, or on the number of cells that release IFN-γ, in T-SPOT®.TB. Laboratories should provide both the qualitative and quantitative results.
- Qualitative results are reported as positive, negative, indeterminate or borderline.
- Quantitative results are reported as numerical values that include a response to the TB antigen and 2 controls, nil and mitogen. Quantitative results may be useful for clinical decision making in individual cases, in combination with risk factors.
Selecting a Test to Detect TB Infection
- IGRAs are the preferred method of testing for:
- Groups of people who have poor rates of return for TST reading and interpretation (e.g., homeless persons)
- Persons who have received BCG vaccination
- TST is the preferred method for testing for:
- Children under the age of 5 years
- Either TST or IGRA may be used without preference for other groups that are tested for LTBI.
Key Point
- Routine testing with both TST and IGRAs is NOT recommended; however, there are certain situations where results from both tests may be useful (See Appendix D).
Special Considerations in Testing for TB Infection
BCG Vaccine
In many parts of the world where TB is common, BCG vaccine is used to protect infants and young children from serious, life-threatening disease, specifically miliary TB and TB meningitis. The World Health Organization (WHO) recommends that BCG vaccine be administered during infancy in TB endemic countries. BCG vaccination is not generally recommended in the United States. The effect of BCG vaccine on TST results often causes confusion. TST reactivity caused by BCG vaccine generally wanes with the passage of time, but periodic skin testing may prolong (boost) reactivity in vaccinated persons. A person with a history of BCG vaccination can be tested and treated for LTBI if they react to the TST. TST reactions should be interpreted based on risk stratification regardless of BCG vaccination history.
IGRAs use M. tuberculosis specific antigens that do not cross react with BCG, and therefore, do not cause false positive reactions in BCG recipients.
HIV Infection
The risk of progression from LTBI to TB disease is 7% to 10% each year for those with both LTBI and untreated HIV infection. Those with LTBI who are not HIV-infected have a 10% risk over their lifetime. Thus the risk of progression to TB disease is 10 times greater for those who are HIV infected. This risk is reduced with antiretroviral therapy for HIV, but is still higher than that in HIV-negative persons with LTBI.
HIV-infected persons should be tested for LTBI as soon as their HIV status becomes known. A negative TST or IGRA result does not exclude LTBI as they may have a compromised ability to react to tests for TB infection. Annual testing should be considered for HIV-infected persons who are TST or IGRA negative on initial evaluation, and who have a risk for exposure to M. tuberculosis. The usefulness of anergy testing in HIV-infected individuals or others has not been demonstrated; therefore, it is not recommended.
After the initiation of antiretroviral therapy (ART), repeat testing for LTBI is recommended for HIV-infected persons previously known to have negative TST or IGRA results. This is because the immune response may be restored by ART.
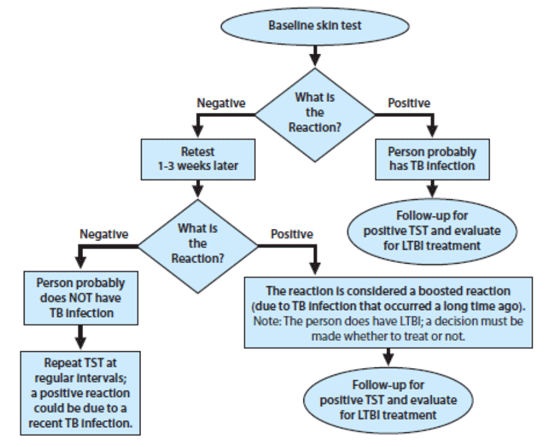
Booster Phenomenon
Some people infected with M. tuberculosis may have a negative reaction to the TST if many years have passed since they became infected. They may have a positive reaction to a subsequent TST because the initial test stimulates their ability to react to the test. This is commonly referred to as the “booster phenomenon” and may incorrectly be interpreted as a skin test conversion (going from negative to positive). For this reason, the “two-step method” is recommended at the time of initial testing for individuals who may be tested periodically (e.g., health care workers). If the first TST result in the two-step baseline testing is positive, consider the person infected and evaluate and treat the person accordingly. If the first test result is negative, the TST should be repeated in 1–3 weeks. If the second test result is positive, consider the person infected and evaluate and treat the person accordingly; if both steps are negative, consider the person uninfected and classify the TST as negative at baseline testing (see Figure 1).
When IGRAs are used for serial testing, there is no need for a second test because boosting does not occur.
Figure 1: Two-Step TST Testing
Contacts
- For contacts of a person with infectious TB disease, retesting in 8–10 weeks after exposure has ended is indicated when the initial TST or IGRA result is negative. In contact investigations, retesting is not called two-step testing. The second test is needed to determine if infection occurred, but was too recent to be detected at the time of the first test.
- Children under the age of 5 years and immunosuppressed persons (e.g., HIV infected) who have negative TST or IGRA results should have a chest radiograph. If chest radiograph is normal, treatment should be started for LTBI and another TST or IGRA performed 8–10 weeks after contact has ended.
- If a repeat TST or IGRA result is positive, treatment should be continued. If it is negative, treatment can usually be discontinued.
- If testing is repeated, the same type of test (TST or IGRA) should be used.
Pregnancy
- TST is both safe and reliable throughout the course of pregnancy.
- Test only if specific risk factors are present for acquiring LTBI or for progression of LTBI to TB disease.
- If a TST or IGRA reaction is positive, obtain a chest radiograph using proper shielding.
Other Diagnostic Considerations
Chest Radiograph
Chest radiographs help differentiate between LTBI and pulmonary TB disease in individuals with positive tests for TB infection. The following guidelines are recommended:
- A chest radiograph should be ordered as part of a medical evaluation for a person who has a positive TST or IGRA result.
- A chest radiograph is also indicated in the absence of a positive test result for TB infection when a person is a close contact of an infectious TB patient and treatment for LTBI will be started (e.g., “window prophylaxis” in a young child or immunocompromised person).
- Children less than 5 years of age should have both posterior-anterior and lateral views; all others should have at least posterior-anterior views.
- Other views or additional studies should be done based on the health care provider’s judgment.
- Persons with nodular or fibrotic lesions consistent with old TB are high-priority candidates for treatment of LTBI after TB disease is excluded.
- Persons with fully calcified, discrete granulomas do not have an increased risk for progression to TB disease.
Sputum Examination for AFB Smear and Culture
Sputum examination is indicated for persons with positive test results for TB infection and either an abnormal chest radiograph or the presence of respiratory symptoms (even when the chest radiograph is normal).
Physical Examination and Medical History
Physical examination and medical history, which includes obtaining information about previous positive tests for TB infection, previous treatment for LTBI or TB disease, and a risk assessment for liver disease, are indicated for an individual with positive TB test results. Written documentation of a previously positive TST or IGRA result is required; a patient’s verbal history is not sufficient. Appendix E provides an example of a documentation form.
- Page last reviewed: October 20, 2014
- Page last updated: November 26, 2014
- Content source:


 ShareCompartir
ShareCompartir