Tuberculosis Epidemiologic Studies Consortium (TBESC)

TBESC II - Striving to Prevent, Control, and Eliminate Tuberculosis
The Tuberculosis Epidemiologic Studies Consortium II (TBESC-2) is a partnership of the Division of Tuberculosis Elimination (DTBE) with academic institutions and TB control programs in 11 states. The Consortium focuses on strategies and tools to increase diagnosis and treatment of latent tuberculosis infection (LTBI) in high-risk populations.
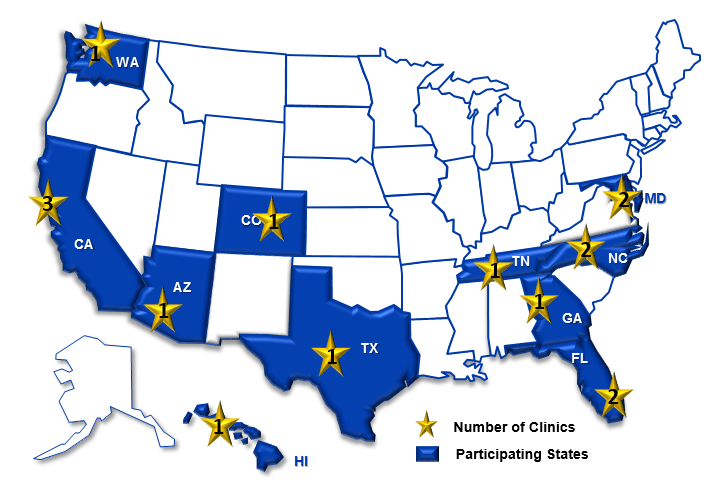
Study Sites
- California Department of Public Health
- Denver Health and Hospital Authority, Colorado
- Duke University, North Carolina and Tennessee
- Emory University, Georgia
- Hawaii Department of Health
- Public Health-Seattle and King County, Washington
- Maricopa County Department of Public Health, Arizona
- Maryland Department of Health and Mental Hygiene
- University of Florida
- University of North Texas Health Science Center
Why Focus on Latent TB Infection?
Mathematical models suggest that identification and treatment of persons with LTBI will have the greatest impact on TB elimination in the United States.
Figures 2 and 3 show the relative impact on TB elimination targets of LTBI treatment for U.S.-born and foreign-born populations. Figure 2 estimates the years to reach the pre-elimination target of <10 cases per million if treatment were doubled or quadrupled from baseline levels. Figure 3 shows the years to reach the elimination target of <1 case per million under the same assumptions.
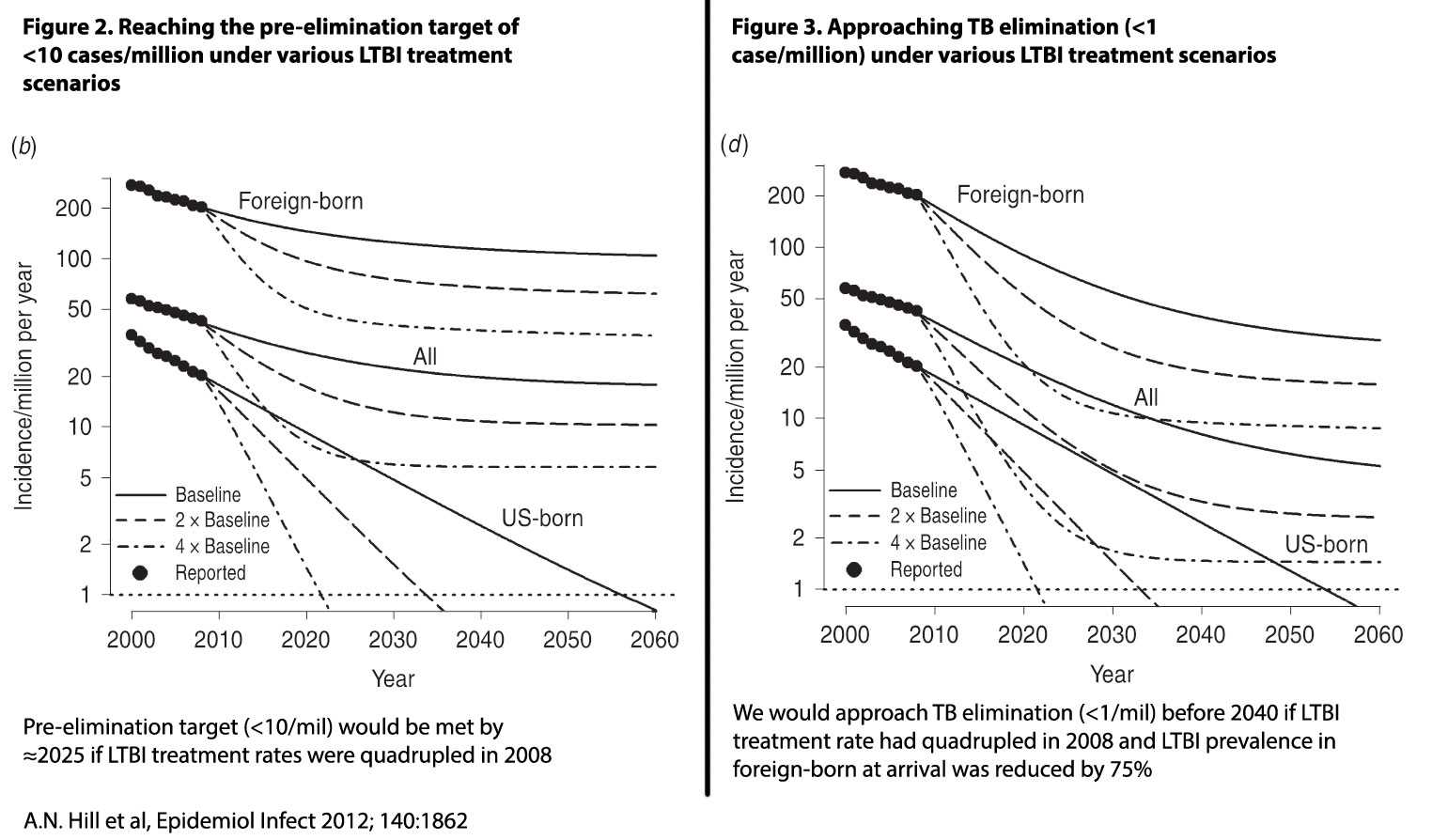
While the figures make clear the importance of identifying and treating persons with LTBI, key activities to achieve those objectives face barriers. These include:
- Lack of a gold standard test for LTBI;
- Insufficient local surveillance data for LTBI; and
- Few proven methodologies to identify and treat high-risk groups.
Study Descriptions
TBESC-II has two main studies, each with several sub studies directed at the key barriers to effective LTBI diagnosis and management.
Study 1: Evaluating tests for LTBI
No definitive diagnostic reference standard (gold standard) exists for LTBI. Three LTBI tests currently are on the market: the tuberculin skin test (TST), developed in 1908; and two recently developed interferon-gamma release assay (IGRA) blood tests-- the QuantiFERON®-TB Gold In-Tube (QFT-GIT), and T-SPOT®.TB test (T-SPOT), approved by the Food and Drug Administration after 2000. All three are indirect tests that measure the body’s immune responsiveness to Mycobacterium tuberculosis proteins. The three tests have varying sensitivities and specificities, and may perform differently in different populations.
This first TBESC-II study is a head-to-head comparison of the three tests in populations at high risk of LTBI and/or progression to TB disease:
- Persons born in or frequent visitors to countries with high rates of TB;
- Close contacts to persons with TB disease; and
- Persons with HIV infection.
When recruitment ends in mid-2017, approximately 21,400 enrollees will have received all three tests; all enrollees are actively followed for two years to identify those who develop TB disease. This study, the largest of its kind to date, will provide more accurate estimates of the sensitivity and specificity of each test and its appropriate use in high-risk populations.
The study is supported by a web-based data management system. The system operates on one platform with modules to oversee enrollment, testing, treatment, and follow-up.
Sub studies
- Repeatability and reproducibility of QFT and T-SPOT
- Feasibility and cost of testing for prediabetes and diabetes in high-risk persons tested for LTBI
- Cost-effectiveness of LTBI testing in high-risk populations
Study 2: The TB Prevention Cascade to Cure
A key barrier to TB elimination in the United States is incomplete knowledge of the burden of M. tuberculosis infection and inadequate preventive services for persons with LTBI. The National Health and Nutrition Examination Survey (NHANES) periodically estimates the prevalence of LTBI among U.S. populations. The most recent estimates from 2012 range from 1.8% of U.S.-born persons to 18.7% of foreign-born persons. However, no programs on a national or local level conduct the type of ongoing and intensive data collection and evaluation of LTBI that is characteristic of the nation’s approach to TB disease.
Such LTBI surveillance programs should include:
- Population-based calculation of LTBI prevalence overall and in high-risk subpopulations;
- Evaluation of changes over time; and
- Identifying where patients are lost to care in the process from LTBI diagnosis to treatment completion.
These program activities comprise the TB Prevention Cascade to Cure, modeled after similar programs that focus intervention efforts for HIV and Hepatitis C. The bar graph below (Figure 4) is a hypothetical example of the TB Prevention Cascade to Cure, with treatment of LTBI as the prevention.
Figure 4: TB Prevention Cascade to Cure: treatment as prevention*
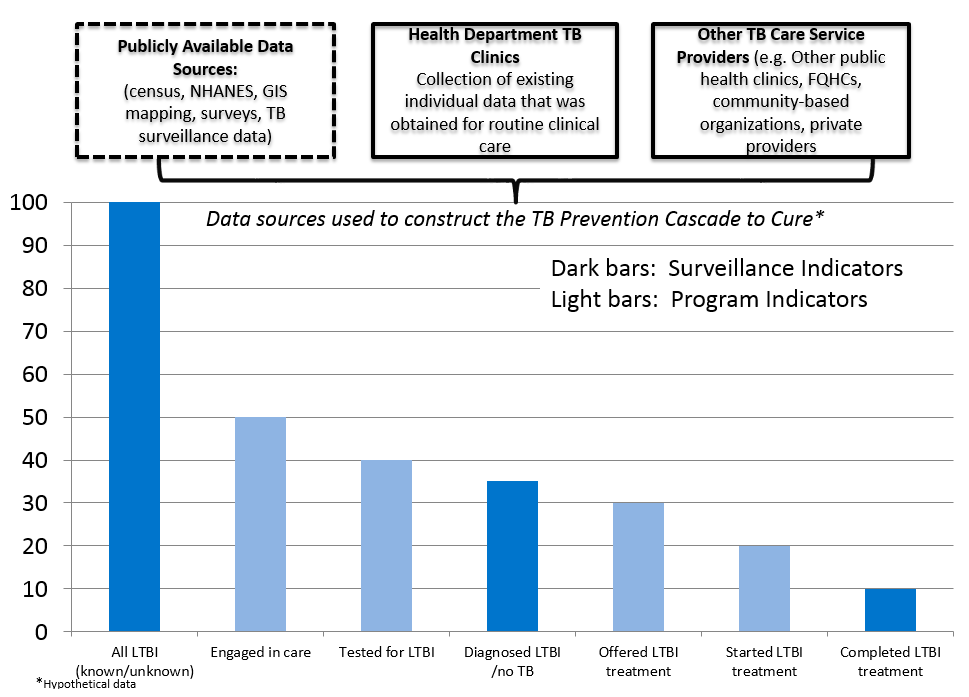
The main goal of the TB Prevention Cascade to Cure study is to develop local estimates of LTBI prevalence in high-risk populations, and the proportions of those with LTBI who are identified, offered treatment, accept treatment, and complete treatment. Once methodologies are developed to calculate these estimates, they will be made available to other interested groups, including TB programs, federally qualified health centers (FQHCs), clinic groups, and clinicians who treat high-risk populations. Identification of these gaps is a first step in developing interventions to successfully close the gaps.
To assist with these efforts, TBESC-II is developing an LTBI surveillance and case management system for TB programs. The Surveillance and TB Elimination Management System (STEMS) will contain modules for patient registration, testing, treatment, follow-up, and contact investigations. TBESC II will use the first versions of this tool to construct the Prevention Cascade to Cure at local sites.
Sub studies
- Develop algorithms to estimate LTBI prevalence at any county or state level.
- Develop tools for local TB programs to identify local clinicians who treat high-risk underserved populations, so they can partner with these groups to increase LTBI treatment.
- Use insurance billing codes to estimate the extent and magnitude of LTBI testing and treatment in commercial and community health sectors.
Study 3: Development and assessment of interventions to close gaps in the Cascade to Cure
Study 3 will focus on the gaps identified in the Cascade to Cure, and the development and testing of interventions to close those gaps. For example, one gap might be that populations at high risk for LTBI are unaware of their risk, and therefore do not seek testing. Possible interventions may include media campaigns; posters and/or flyers in social service offices and medical facilities that see large numbers of high-risk persons; and training of community members and service professionals who interact with high-risk populations.
Study 4: Clinical trial to assess a shorter regimen for LTBI treatment
Although six to nine months of isoniazid (INH) therapy is highly effective (60-90%) in preventing progression of LTBI to TB disease, the long duration of therapy hinders treatment completion. Recent trials have shown a 12-week regimen of INH and rifapentine (3HP) has equivalent effectiveness with higher completion rates (above 80%). Study 4, a collaboration with the DTBE-sponsored Tuberculosis Trials Consortium and the British Medical Research Council, will compare short course rifampin-based regimens such as 3HP with an even shorter regimen: six weeks of daily rifapentine. Researchers hope the results of this study will expand the LTBI treatment tools available to clinicians.
TBESC I
TBESC- 2’s predecessor, TBESC-1, conducted programmatically relevant epidemiologic, behavioral, economic, laboratory, and operational research for TB prevention and control. TBESC-1 conducted 29 research studies, ranging from the molecular epidemiology of multidrug-resistant TB to the cost of TB in urban health departments.
- Page last reviewed: March 31, 2017
- Page last updated: March 31, 2017
- Content source:


 ShareCompartir
ShareCompartir