TB NOTES

TB Notes 5December 2016
Notes from the Director
Dear Colleague:
This fall has been a busy one for DTBE. The September release of the U.S. Preventive Services Task Force (USPSTF) recommendation provides a new opportunity for the TB community to draw attention to latent TB infection and educate the public, health care providers, at-risk populations, and policy makers on the importance of targeted testing and treatment for latent TB infection.
DTBE staff were involved in several conferences in September and October, including the September Tuberculosis Education and Training Network (TB ETN) and the Tuberculosis Program Evaluation Network (TB PEN) Conference, the International Union against TB and Lung Disease conference in October, and the TB Program Managers’ Course in October. In addition, we released the 2015 Annual Surveillance Report in November.
These activities, and the many others not mentioned here, continue to demonstrate DTBE’s commitment to fulfill its mission to promote health and quality of life by preventing, controlling, and eventually eliminating tuberculosis in the United States. As we enter the last month of the year, I continue to be inspired by the great work you do every day. I wish you all safe and enjoyable holidays, and look forward to the great things we can accomplish together in 2017.
Philip LoBue, MD, FACP, FCCP
Director,
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
TB Education and Training Network (TB ETN) Updates
2016 TB Education and Training Network (TB ETN) Award Winners
The 2016 TB ETN/PEN Conference marked the sixth year for the TB Educator of the Year Award and TB Project Excellence Award. These awards were established in 2010 to recognize excellence in TB health education and training by TB ETN members around the world.
The TB Educator of the Year award recognizes an individual who has shown dedication and leadership in the field of TB education and training. There were many excellent nominations and two winners were selected for this year’s award: Denise Dodge and Renee Simmons-Wilkins.
 Denise Dodge is the Assistant Director and Nurse Consultant for TB Control at the Virginia Department of Health. Denise is in charge of responding to clinical questions from nurses in more than 80 local health departments. She also creates and provides trainings for all of the new TB nurses. Previously, she was employed as a Nurse Consultant at the Southeastern National TB Center. Denise’s life work has been to train and educate everyone she meets on the importance of TB prevention and control in the most enjoyable way possible. She has been described as “uniquely gifted” and a “powerhouse,” and has made a tremendous impact in the TB community.
Denise Dodge is the Assistant Director and Nurse Consultant for TB Control at the Virginia Department of Health. Denise is in charge of responding to clinical questions from nurses in more than 80 local health departments. She also creates and provides trainings for all of the new TB nurses. Previously, she was employed as a Nurse Consultant at the Southeastern National TB Center. Denise’s life work has been to train and educate everyone she meets on the importance of TB prevention and control in the most enjoyable way possible. She has been described as “uniquely gifted” and a “powerhouse,” and has made a tremendous impact in the TB community.
 Renee Simmons-Wilkins is a master trainer/disease investigator who has worked in the field of communicable disease investigation since 1994. Renee currently serves as course faculty for the Curry International TB Center, where she receives rave reviews from training participants. Comments from participants note how they enjoy Renee’s engaging teaching style and wonderful spirit. Renee has made tremendous contributions to the field of TB education and training, and is one of the most respected and valued members of Curry International TB Center’s esteemed faculty.
Renee Simmons-Wilkins is a master trainer/disease investigator who has worked in the field of communicable disease investigation since 1994. Renee currently serves as course faculty for the Curry International TB Center, where she receives rave reviews from training participants. Comments from participants note how they enjoy Renee’s engaging teaching style and wonderful spirit. Renee has made tremendous contributions to the field of TB education and training, and is one of the most respected and valued members of Curry International TB Center’s esteemed faculty.
 The Project Excellence award recognizes exceptional health education and training products or activities that have been developed by TB ETN members within the past 2 years. The 2016 TB Project Excellence Award goes to the “Child TB Project.” This project was developed by the Bangladesh Pediatric Association; the lead for the project was Dr. Shakil Ahmed.
The Project Excellence award recognizes exceptional health education and training products or activities that have been developed by TB ETN members within the past 2 years. The 2016 TB Project Excellence Award goes to the “Child TB Project.” This project was developed by the Bangladesh Pediatric Association; the lead for the project was Dr. Shakil Ahmed.
Bangladesh is the 6th highest TB-burdened country in the world, and childhood TB is considerably under reported. Over 10 months, the TBCARE II/USAID funded project targeted health care providers in the Dhaka Division of Bangladesh.
Using principles of adult learning and systematic health education, the Child TB Project Team developed training modules and materials to improve skills in screening, diagnosis, and management of child TB. The training consisted of interactive sessions, assigned readings, role playing, group work, case studies, and center visits. The Child TB Project Team led workshops for over 9,500 health care providers at three levels of service delivery including clinicians, community health workers, and facility managers.
Congratulations to all of the 2016 award winners!
Submitted by Peri Hopkins, MPH, DTBE
Communications, Education, and Behavioral Studies Branch Updates
Education & Evaluation: Driving Toward TB Elimination
The Tuberculosis Education and Training Network (TB ETN) and the Tuberculosis Program Evaluation Network (TB PEN) Conference was held September 20-22, 2016 at the CDC Global Communications Center in Atlanta, Georgia. The theme of the 2016 conference was Education and Evaluation: Driving Toward TB Elimination. The TB ETN/PEN Conference emphasized the systematic health education process and program evaluation planning and implementation. Conference activities included skills-based workshops, informational presentations, and networking opportunities. The 240 participants included TB education & training and TB program evaluation specialists from TB programs throughout the United States and U.S. affiliated Pacific Islands, as well as federal and international TB program staff.
The two-and-a-half-day conference included a variety of dynamic plenary and breakout sessions aimed at skill building and information sharing. The opening plenary presentation was delivered by Dr. Phil LoBue, Director of the Division of Tuberculosis Elimination at CDC. Dr. LoBue discussed latent TB infection (LTBI) surveillance and the newly released LTBI screening recommendation from the U.S. Preventive Services Task Force. He emphasized the importance of TB education and evaluation, and expressed his support for strong TB education and evaluation activities in state and local TB programs. Other conference highlights included sessions on how to educate policy makers about TB, intermediate evaluation planning and implementation, LTBI surveillance, tools for caring for TB patients who have diabetes, economic evaluation, technology to enhance cohort review, and video/electronic directly observed therapy practices. Speakers included experts in TB education, training, and evaluation at the local, state, national, and international levels.
The conference also hosted a scientific poster session with 34 abstracts accepted for poster presentations. Authors of four of the submitted abstracts (two focused on TB education & training, and two focused on TB program evaluation) were invited to give oral presentations. The four abstracts that were selected for oral presentations were:
- “An Evaluation of the Use of Video Technology in Directly Observed Therapy (VDOT) for the Treatment of Tuberculosis (TB),” presented by Kristin St John (Rhode Island);
- “Training Nurses in Tuberculosis in the Dialysis Setting,” presented by Pay Iyer (Massachusetts);
- “Evaluation of the Michigan Tuberculosis Cohort Review: Identification of Unrecognized Barriers and Uptake in Incentive and Enabler Use,” presented by Helen McGuirk (Michigan); and
- “Partnerships for TB Elimination: the Central Role of Training and Education,” presented by Cathy Miller (California).
Submitted by Peri Hopkins, MPH, DTBE and Rachel Yelk Woodruff, MPH, DTBE
TB Program Managers’ Course 2016
The overall purpose of the TB Program Managers’ Course is to improve the planning and managerial capabilities of new TB program managers throughout the country. The course is designed for TB controllers, program managers, public health advisors, and nurse consultants with programmatic responsibilities at the state, big city, territory, or regional (within a state) level. Course participants should have occupied a TB program management position for at least 6 months, but no more than 3 years, and are nominated by the DTBE Program Consultant for their area.
The Communications, Education, and Behavioral Studies Branch (CEBSB) would like to thank the faculty and participants of the 2016 TB Program Managers’ Course for making this year’s course such a success. The hard work of the faculty in preparing the materials for their sessions and the participants’ hard work during the course are greatly appreciated.
 The course was held October 24-27, 2016 at CDC’s Global Communications Center. The 4-day in-person training was divided into 19 sessions. The course stressed practical application of planning, management, and evaluation concepts to the specific issues and concerns of TB programs. Skills essential to TB program management were presented, followed by exercises and polling questions that encouraged participants to use knowledge learned.
The course was held October 24-27, 2016 at CDC’s Global Communications Center. The 4-day in-person training was divided into 19 sessions. The course stressed practical application of planning, management, and evaluation concepts to the specific issues and concerns of TB programs. Skills essential to TB program management were presented, followed by exercises and polling questions that encouraged participants to use knowledge learned.
For the participants, the course is not entirely over. They will be mailed a 6-month follow-up evaluation in April 2017. Once this evaluation is completed and returned, each participant will receive a certificate of completion for the course.
Submitted by Molly Dowling, MPH, DTBE
CDC Announces World TB Day 2017 Theme: End TB
 World TB Day, observed on March 24, provides the opportunity to raise awareness about TB-related problems and solutions and to support worldwide TB-control efforts. CDC will join our international partners by promoting the global Stop TB Partnership’s 2017 World TB Day theme, End TB, which was the same theme featured in 2016. CDC’s World TB Day website will be updated with 2017 information, materials, and resources. If you have questions, comments, or ideas for World TB Day 2017, please contact Leeanna Allen at lallen1@cdc.gov.
World TB Day, observed on March 24, provides the opportunity to raise awareness about TB-related problems and solutions and to support worldwide TB-control efforts. CDC will join our international partners by promoting the global Stop TB Partnership’s 2017 World TB Day theme, End TB, which was the same theme featured in 2016. CDC’s World TB Day website will be updated with 2017 information, materials, and resources. If you have questions, comments, or ideas for World TB Day 2017, please contact Leeanna Allen at lallen1@cdc.gov.
Submitted by Leeanna Allen, MPH, DTBE
Laboratory Branch Updates
The Applied Research Team expands capacity for whole genome sequencing
Since 2004, DTBE has conducted molecular surveillance of Mycobacterium tuberculosis (Mtb) nationally. Genotyping data are integrated with patient demographic and clinical data, and routinely analyzed to identify suspected outbreaks. The discriminatory power of conventional genotyping methods is sometimes insufficient; some suspected outbreaks identified by genotyping include a mix of outbreak and sporadic cases, or do not represent an outbreak at all. Genomic surveillance has the power to increase the accuracy of outbreak detection systems and to help stop the transmission of tuberculosis.
In collaboration with the Surveillance, Epidemiology, and Outbreak Investigations Branch (SEOIB), the DTBE Laboratory Applied Research Team has investigated and performed whole genome sequencing (WGS) on approximately 80 different clusters of TB cases representing more than 1,000 isolates. Once completed and analyzed, the results and interpretation of the data are shared with state and local TB programs; our experiences and feedback from these programs demonstrate that WGS data result in a more focused use of limited public health resources, leading to more effective epidemiologic field investigations and an improved ability to identify where public health intervention will have the greatest impact.
In 2016, the Applied Research Team led by Dr. James Posey was awarded a grant from the CDC Office of Advanced Molecular Detection to expand the capacity for WGS of Mtb. In collaboration with the Association of Public Health Laboratories (APHL), DTBE funded 5 state laboratories to provide WGS of approximately 200 Mtb isolates per month. The data and information generated from this project will help us obtain the knowledge necessary to successfully implement genomic surveillance of Mtb at the national level, build state capacity to conduct WGS, and work with state and local partners to use these results to guide field investigations and direct public health resources more effectively than ever before.
Submitted by James Posey, PhD, DTBE
Surveillance Epidemiology and Outbreak Investigations Branch Updates
The 2015 Annual Surveillance Report is here!
The report, entitled Reported Tuberculosis in the United States, 2015, is now available online.
Key findings:
- There were 9,557 TB cases reported in the United States in 2015, which represents a 1.6% increase from 2014.
- The overall annual TB incidence remained level at approximately 3.0 cases per 100,000.
- People born outside of the United States continue to bear the burden of TB, largely because of reactivation of latent TB infection that occurred in their country of origin.
The first increase in TB cases in the United States in 23 years underscores the need for more comprehensive public health approaches in TB prevention and control. Eliminating TB in the United States requires an expanded approach to test and treat latent TB infection, and strengthen existing systems to stop TB transmission.
DTBE has developed a slide set, fact sheet, infographic, and web graphics with highlights from the surveillance report to support TB education and outreach to clinicians, health care agencies, and community organizations.
Submitted by CEBSB and SEOIB, DTBE
The Analytic Steering Committee (ASC) and how Data are used for Research
Over the past 13 years, the Analytic Steering Committee (ASC) has invited proposals from investigators interested in analyzing TB surveillance data, and ensured the excellence and prompt publication of analyses of these data. These publications are used by DTBE staff, state TB personnel, other CDC staff, and the general public. They inform and educate the general public on research done to prevent, control, and eventually eliminate TB.
Below is a brief summary of the ASC accomplishments, and information about the proposal submission process for potential investigators.
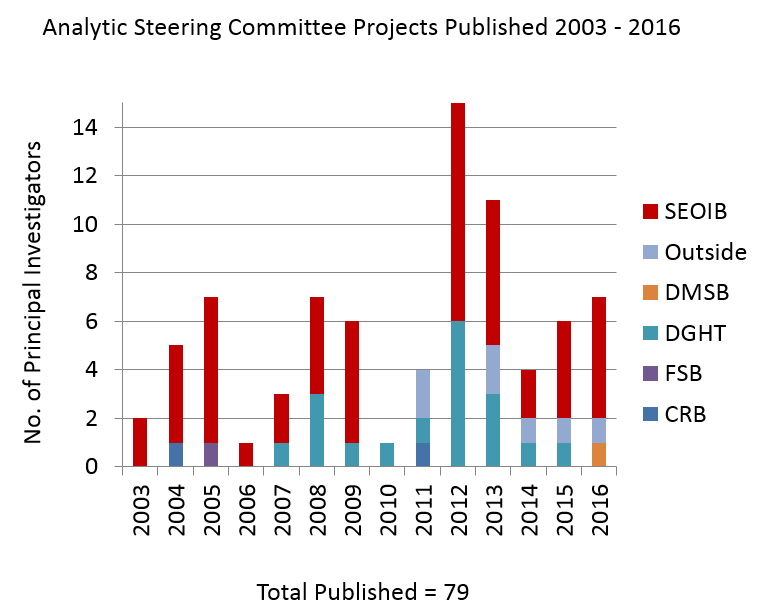
During its tenure, the ASC has received and reviewed 159 proposals. Of these, 79 have resulted in publications.
The bar chart to the right shows the number of the ASC projects published since 2003, and their principal investigator’s affiliations (DTBE, other CDC, and outside CDC).
Currently there are 15 projects in the analysis phase, 19 manuscripts in progress, and 6 projects nearing the final stages of publication.
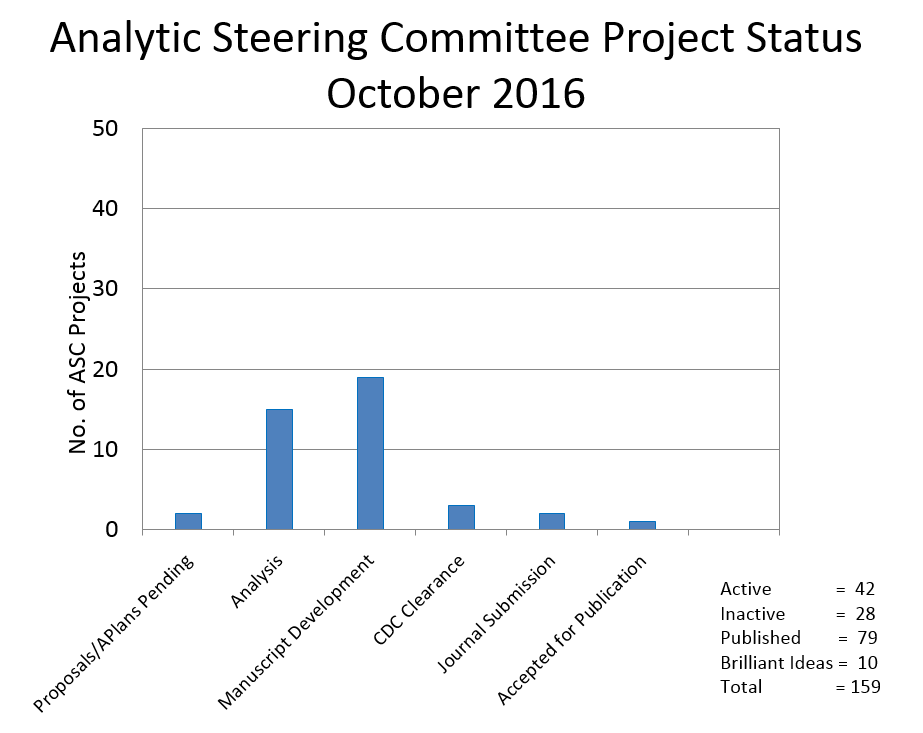
Another 4 projects are on hold at this time. Ten projects were placed on the “Brilliant Ideas List” due to principal investigators’ time constraints. These ideas are available to other investigators.
There are a total of 22 projects that were either ‘not published’, ‘not approved by the ASC’, ‘withdrawn’, ‘not completed’ or ‘terminated.’
The bar chart to the left shows the current status of projects.
Prior to 2003, there were 13 proposals grandfathered that existed prior to the creation of the ASC. Of these 13 proposals, 10 (77%) were published. Some of these manuscripts include:
Between 2003 to early October 2016, the ASC received 146 proposals/analytic plans. Of the 146 proposals/analytic plans, 69 (47.3%) of the manuscripts have been published. Some of these manuscripts include:
Below is a brief overview of the ASC review and approval process. For more information about the process contact Glenda Newell (GNewell@cdc.gov) or Adam Langer ALanger@cdc.gov)
The ASC reviews requests for patient-level data to ensure that proposed analyses:
- Constitute unique contributions to the scientific body of knowledge that do not duplicate ongoing or previously completed analyses
- Are consistent with established policies for the use of TB surveillance data (for more information about the established policies contact Glenda Newell GNewell@cdc.gov or Adam Langer ALanger@cdc.gov)
- Propose appropriate research questions and analytic methodologies
ASC Process
The Key Milestones of the ASC process are project proposal/analytic plan, preliminary results and conclusions, and final manuscript. To obtain project approval, a proposal/analytic plan, including hypothesis generation and data needs (e.g., RVCT data only, or Census data), must be submitted to the Steering Committee. Approval is granted or denied. Following ASC approval and before receiving access to these data, all investigators to be given access to these data must have completed the web-based Assurance of Confidentiality training within the past year; they must also renew it annually, as long the investigators continue to have access to surveillance data.
Following completion of the Assurance of Confidentiality training, the Surveillance Team will provide access to surveillance data for analysis in a manner consistent with the security and confidentiality practices in place at the time that the data are made available.
For the final key milestone, a manuscript is submitted for clearance at the NCHHSTP center level. After manuscript is cleared, Principal Investigator submits to journal for publication.
The Analytic Steering Committee (ASC) Division of Tuberculosis Elimination positions are:
Team Lead, Surveillance Team, SEOIB (ASC Chair)
SEOIB Branch Chief
DTBE Associate Director for Science
Molecular Epidemiology Activity Lead, SEOIB
Team Lead, Data Management Statistics Branch
ASC Coordinator, SEOIB
Submitted by Glenda Newell, DTBE
New CDC Publications
August 2016
Auld SC, Lee SH, Click ES, Miramontes R, Day CL, Gandhi NR, Heilig CM. Interferon Gamma Release Assay Result is Associated with Disease Site and Death in Active Tuberculosis. Ann Am Thorac Soc. 2016 Aug 31. PMID: 27580246. [Epub ahead of print.]
Shah NS, Grace Lin SY, Barry PM, Cheng YN, Schecter G, Desmond E. Clinical Impact on Tuberculosis Treatment Outcomes of Discordance Between Molecular and Growth-Based Assays for Rifampin Resistance, California 2003-2013. Open Forum Infect Dis. 2016 Aug 24;3(3):ofw150. PMID: 27704008.
September 2016
Kaniga K, Cirillo DM, Hoffner S, Ismail NA, Kaur D, Lounis N, Metchock B, Pfyffer GE, Venter A. A Multi-Laboratory, Multi-Country Study to Determine Bedaquiline Minimal Inhibitory Concentration Quality Control Ranges for Phenotypic Drug-Susceptibility Testing. J Clin Microbiol. 2016 Sep 21; pii: JCM.01123-16. PMID: 27654337. [Epub ahead of print.]
Kaniga K, Cirillo DM, Hoffner S, Ismail NA, Kaur D, Lounis N, Metchock B, Pfyffer GE, Venter A. A Multi-Laboratory, Multi-Country Study to Determine Minimal Inhibitory Concentration Quality Control Ranges for PhenotypicDrug-Susceptibility Testing of Selected First-Line Anti-TB Drugs, Second-Line Injectables, Fluoroquinolones, Clofazimine and Linezolid. J Clin Microbiol. 2016 Sep 21; pii: JCM.01138-16. PMID: 27654338. [Epub ahead of print.]
Lambert LA, Armstrong LR, Lobato MN, Ho C, France AM, Haddad MB. Tuberculosis in Jails and Prisons: United States, 2002-2013. Am J Public Health. 2016 Sep 15:e1-e7. PMID: 27631758. [Epub ahead of print.]
Mangan JM, Tupasi TE, Garfin AM, Lofranco V, Orillaza-Chi R, Basilio R, Naval LC, Balane GI, Joson ES, Burt D, Lew WJ, Mantala M, Pancho S, Sarol JN, Golubkov A, Kurbatova EV. Multidrug-resistant tuberculosis patients lost to follow-up: self-reported readiness to restart treatment. Int J Tuberc Lung Dis. 2016 Sep;20(9):1205-11. doi: 10.5588/ijtld.16.0029. PMID: 27510247.
Olea-Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher-Vindel E, Forcella S, Silk BJ, Ditiu L, El Idrissi A, Raviglione M, Cosivi O, LoBue P, Fujiwara PI. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis-a call for action. Lancet Infect Dis. 2016 Sep 30; pii: S1473-3099(16)30139-6. doi: 10.1016/S1473-3099(16)30139-6. Review. PMID: 27697390. [Epub ahead of print.]
October 2016
Auld AF, Agizew T, Pals S, Finlay A, Ndwapi N, Boyd R, Alexander H, Mathoma A, Basotli J, Gwebe-Nyirenda S, Shepherd J, Ellerbrock TV, Date A. Implementation of a pragmatic, stepped-wedge cluster randomized trial to evaluate impact of Botswana’s Xpert MTB/RIF diagnostic algorithm on TB diagnostic sensitivity and early antiretroviral therapy mortality. BMC Infect Dis. 2016 Oct 26;16(1):606. PMID: 27782821.
Fletcher R, Jones JD, Shah NS. Treatment of Active Tuberculosis in Chicago, 2008-2011: The Role of Public Health Departments. PLoS One. 2016 Oct 12;11(10):e0164162. doi: 10.1371/journal.pone.0164162. PMID: 27732650.
Jayakumar A, Savic RM, Everett CK, Benator D, Alland D, Heilig CM, Weiner M, Friedrich SO, Martinson NA, Kerrigan A, Zamudio C, Goldberg SV, Whitworth WC, Davis JL, Nahid P. Xpert MTB/RIF Assay Shows Faster Clearance of Mycobacterium tuberculosis DNA with Higher Levels of Rifapentine Exposure. J Clin Microbiol. 2016 Dec;54(12):3028-3033. doi: 10.1128/JCM.01313-16. PMID: 27733634. Epub 2016 Oct 12.
Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O’Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016 Oct 1;63(7):853-67. doi: 10.1093/cid/ciw566. PMID: 27621353.
Peloquin CA, Dorman SE, Vernon A, Battista Migliori G, Nahid P. Reply to Alffenaar et al. Clin Infect Dis. 2016 Oct 26. pii: ciw679. No abstract available. PMID: 27789610. [Epub ahead of print.]
Teeter LD, Vempaty P, Nguyen DT, Tapia J, Sharnprapai S, Ghosh S, Kammerer JS, Miramontes R, Cronin WA, Graviss EA; Tuberculosis Epidemiologic Studies Consortium. Validation of genotype cluster investigations for Mycobacterium tuberculosis: application results for 44 clusters from four heterogeneous United States jurisdictions. BMC Infect Dis. 2016 Oct 21;16(1):594. PMID: 27769182.
November
Mindra G, Wortham JM, Haddad MB, Salinas JL, Powell KM, Armstrong LR. Tuberculosis Among Incarcerated Hispanic Persons in the United States, 1993-2014. J Immigr Minor Health. 2016 Nov 29. PMID: 27900592. [Epub ahead of print].
- Page last reviewed: December 13, 2016
- Page last updated: December 13, 2016
- Content source:


 ShareCompartir
ShareCompartir