Tuberculosis Trials Consortium (TBTC)
 The TBTC is a collaboration of researchers from the CDC, domestic and international public health departments, academic medical centers, and selected Veterans Administration medical centers whose mission is to conduct programmatically relevant research concerning the diagnosis, clinical management, and prevention of tuberculosis (TB) infection and disease. The purposes of the TBTC are to conduct research that expands clinical and epidemiologic knowledge of TB and facilitates the diagnosis, clinical management, and prevention of tuberculosis infection and disease; to integrate research into the care of persons with TB infection and disease; to develop research questions that are relevant to program settings in general; to promote research within local TB control programs through collaboration on clinical research of relevance to public health settings; and to provide a forum for international collaborative research of importance to both domestic and international TB control.
The TBTC is a collaboration of researchers from the CDC, domestic and international public health departments, academic medical centers, and selected Veterans Administration medical centers whose mission is to conduct programmatically relevant research concerning the diagnosis, clinical management, and prevention of tuberculosis (TB) infection and disease. The purposes of the TBTC are to conduct research that expands clinical and epidemiologic knowledge of TB and facilitates the diagnosis, clinical management, and prevention of tuberculosis infection and disease; to integrate research into the care of persons with TB infection and disease; to develop research questions that are relevant to program settings in general; to promote research within local TB control programs through collaboration on clinical research of relevance to public health settings; and to provide a forum for international collaborative research of importance to both domestic and international TB control.
Since its inception, the Tuberculosis Trials Consortium (TBTC) has undertaken nine major trials and 15 sub-studies. The completed TBTC studies have resulted in 25 publications in peer-reviewed journals, as well as over 100 presentations, posters, and abstracts at major medical or scientific meetings. The results of TBTC Study 22 significantly influenced the most recent American Thoracic Society (ATS)/CDC guidelines for treatment of TB (published in 2003); other TBTC study results have led to modification of CDC’s recommendations for treatment of TB and HIV.
- Study Descriptions
- Publications and Presentations (PDF - 135K)
- Background
In 2009 the TBTC underwent its third formal external re-competition. In the next decade (2010–2020), TBTC patient enrollment will shift from clinical sites located mostly in North America to sites which are predominantly international. The 2009 re-competition has expanded TBTC’s international presence from a few clinical study sites located outside of North America, to sites in Peru, Spain, South Africa (two sites), Uganda, Kenya, Vietnam, and China (Hong Kong). The TBTC 2010–2020 research group also includes U.S. sites in New York, Washington DC, Texas (four sites), Colorado and Tennessee. Some TBTC sites in and outside North America are linked, in that CDC awarded funds for the international study sites to the U.S.-based institutions that proposed them as partners in the competitive process.
TBTC clinical trials have enrolled more than 14,000 patients and volunteers over the past 20 years. The consortium's annual operating budget is approximately $9,000,000.00. Confronting the many challenges to the successful development of new TB drugs and treatment regimens, the TBTC looks forward with optimism. The late pipeline of new anti-TB drug candidates is the most promising in 40 years, and advances in TB clinical trials science have fostered the progress of these agents. With commitment and support from CDC, the TBTC provides a unique resource for these clinical studies, and will continue to play an important role in improving TB treatment, prevention and control. Currently the TBTC is conducting an international, multicenter, randomized, controlled, open-label, 3-arm, phase 3 non-inferiority trial. The results of this clinical trial determine the efficacy, using the definitive endpoint of durable cure, of a regimen containing rifapentine substituted for rifampin and administered in combination with other drugs for 17 weeks (approximately four months).
The infrastructure of the TB Trials Consortium includes:
• A network of clinical sites worldwide whose principal investigators are recognized experts in tuberculosis treatment and prevention
• Experienced clinical coordinators and outreach workers at each of the funded sites
• Extensive communications systems, including biannual conferences
• Close and collaborative relationships with local TB control programs to facilitate the recruitment and management of trial patients
• An expert Data & Safety Monitoring Board, which reviews active protocols
• Coordination with the CDC IRB and local IRBs, and support for a CDC-based Central IRB process
• A Data and Coordinating Center at the Centers for Disease Control and Prevention (CDC) in Atlanta
• Cooperative relationships with key manufacturers of TB drugs
• Support for monitoring, training, and protocol development from a leading international contract research organization
• Laboratory support from the national reference laboratory housed in the Mycobacteriology Laboratory Branch at CDC
Developing new TB treatment and prevention strategies depends upon collaboration among academic, private sector and government researchers and non-governmental organizations. CDC works closely with such organizations as the Food and Drug Administration, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID), the Global Alliance for TB Drug Development (TB Alliance), the Foundation for Innovative New Diagnostics (FIND), the Johns Hopkins Center for Tuberculosis Research, the TB Research Unit at Case Western Reserve University, commercial drug manufacturers, and others within and outside the United States.
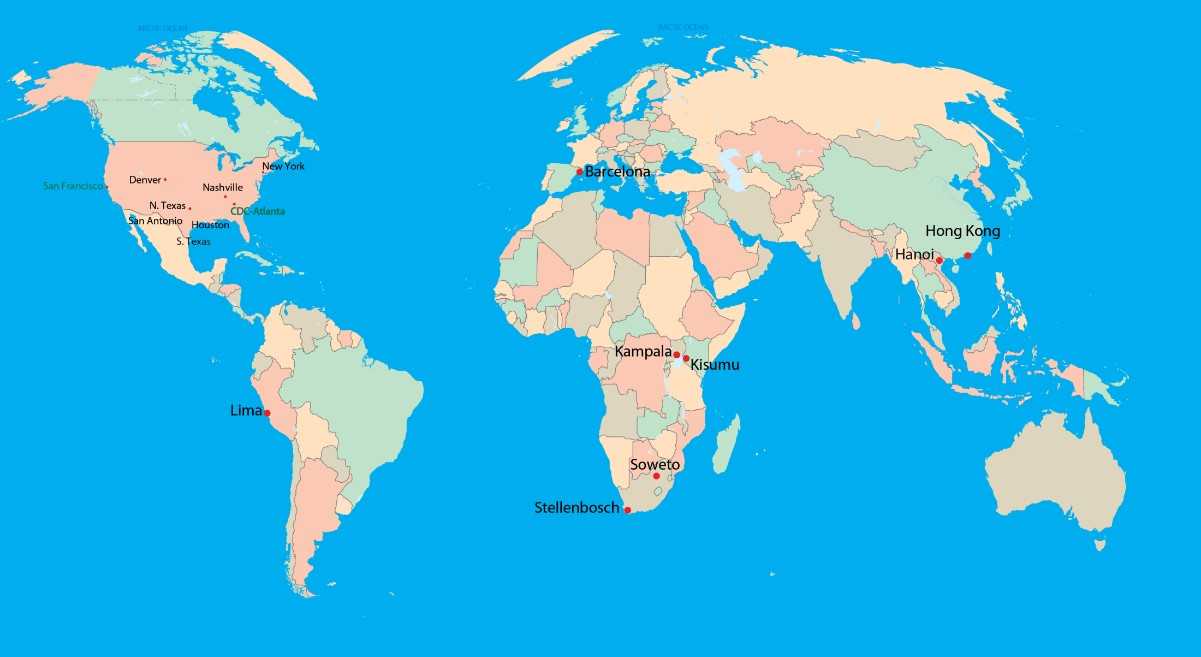
TBTC Study Sites and Partner Institutions 2010-2020
TBTC sites are located in the US, Peru, Spain, South Africa, Uganda, Kenya, Vietnam, and China.
In addition the map shows affiliation of international sites with universities or medical centers in the US:
- site in Barcelona, Spain, is affiliated with University of North Texas
- site in Soweto, South Africa, is affiliated with Johns Hopkins University
- site in Hong Kong, China, is affiliated with Denver Public Health Department
- site in Hanoi, Vietnam, is affiliated with University of California in San Francisco
- site in Lima, Peru, is affiliated with Vanderbilt University

- Page last reviewed: March 27, 2017
- Page last updated: March 27, 2017
- Content source:


 ShareCompartir
ShareCompartir