Appendix F2 - Figure 4
Supplement F: Laboratory Guidance
Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS) Version 2/3
Figure 4 - Serologic Confirmatory Testing
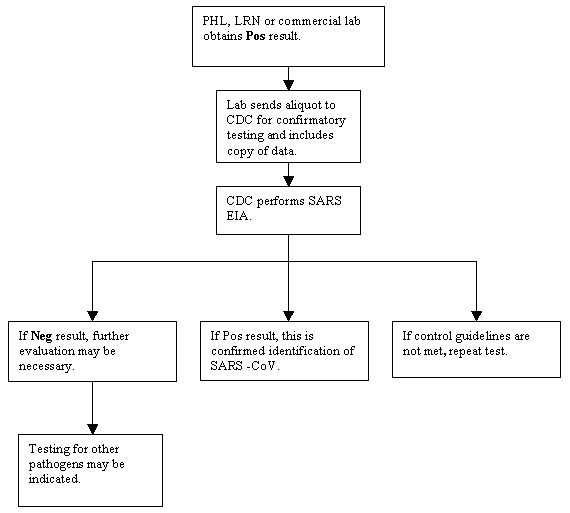
Text version of Figure 4
PHL, LRN or commercial lab obtains Pos result.
Lab sends aliquot to CDC for confirmatory testing and includes copy of data.
CDC performs SARS EIA.
- If Pos result, this is confirmed identification of SARS-CoV.
- If control guidelines not met, repeat test.
- If Neg result, further evaluation may be necessary.
- Testing for other pathogens may be indicated.
Related Pages
- Summary
- I. Rationale and Goals
- II. Lessons Learned
- III. Diagnostic Assays
- IV. CDC's Laboratory Diagnostics Plan
- Appendix F1: Proficiency Testing for Public Health Laboratories Performing SARS-CoV EIA and RT-PCR Diagnostics
- Appendix F2: SARS-CoV Specimen Testing Guidelines: RT-PCR and Serology
- Appendix F3: Guidelines for Clinicians: The Consent Process for SARS-CoV RT-PCR and EIA Testing at CDC and Public Health Laboratories
- Appendix F4: Guidelines for Collecting Specimens from Potential SARS Patients
- Appendix F5: Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with SARS-CoV
- Appendix F6: Guidelines for Medical Surveillance of Laboratory Personnel Working with SARS-CoV
- Appendix F7: Fact Sheet for Clinicians: Interpreting SARS-CoV Test Results from CDC and Other Public Health Laboratories
- Appendix F8: Guidelines for Laboratory Diagnosis of SARS-CoV Infection
- Page last reviewed: May 3, 2005
- Page last updated: May 3, 2005
- Content source:


 ShareCompartir
ShareCompartir