Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness (version 2)
Printer friendly version [13 pages]
NOTICE
Since 2004, there have not been any known cases of SARS reported anywhere in the world. The content in this Web site was developed for the 2003 SARS epidemic. But, some guidelines are still being used. Any new SARS updates will be posted on this Web site.
This document provides guidance on the clinical evaluation and management of patients who present from the community with fever and/or respiratory illnesses. Recommendations supercede those in the Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
Summary of Changes in Version 2
This updated version of the clinical guidance clarifies that, in a setting of ongoing SARS-CoV transmission in a facility or community, the presence of either fever or lower respiratory symptoms should prompt further evaluation for SARS-CoV disease. In addition, in accordance with the new SARS case definition, when persons have a high risk of exposure to SARS-CoV (e.g., persons previously identified through contact tracing or self-identified as close contacts of a laboratory-confirmed case of SARS-CoV disease; persons who are epidemiologically linked to a laboratory-confirmed case of SARS-CoV disease), the clinical screening criteria should be expanded to include, in addition to fever or lower respiratory symptoms, the presence of other early symptoms of SARS-CoV disease.
I. Introduction
Severe acute respiratory syndrome (SARS) is a recently recognized febrile severe lower respiratory illness that is caused by infection with a novel coronavirus, SARS-associated coronavirus (SARS-CoV). During the winter of 2002 through the spring of 2003, WHO received reports of >8,000 SARS cases and nearly 800 deaths. No one knows if SARS-CoV transmission will recur, but it is important to be prepared for that possibility. Early recognition of cases and application of appropriate infection control measures will be critical in controlling future outbreaks.
Key Concepts
- The vast majority of patients with SARS-CoV disease 1) have a clear history of exposure either to a SARS patient(s) or to a setting in which SARS-CoV transmission is occurring, and 2) develop pneumonia.
- Laboratory tests are can be helpful but do not reliably detect infection early in the illness.
Many studies have been undertaken or are underway to evaluate whether there are specific laboratory and/or clinical parameters that can distinguish SARS-CoV disease from other febrile respiratory illnesses. Researchers are also working on the development of laboratory tests to improve diagnostic capabilities for SARS-CoV and other respiratory pathogens. To date, however, no specific clinical or laboratory findings can distinguish with certainty SARS-CoV disease from other respiratory illnesses rapidly enough to inform management decisions that must be made soon after the patient presents to the healthcare system. Therefore, early clinical recognition of SARS-CoV disease still relies on a combination of clinical and epidemiologic features.
II. Identification of Potential Cases of SARS-CoV Disease
The diagnosis of SARS-CoV disease and the implementation of control measures should be based on the risk of exposure. In the absence of any person-to-person transmission of SARS-CoV worldwide, the overall likelihood that a patient being evaluated for fever or respiratory illness has SARS-CoV disease will be exceedingly low unless there are both typical clinical findings and some accompanying epidemiologic evidence that raises the suspicion of exposure to SARS-CoV. Therefore, one approach in this setting would be to consider the diagnosis only for patients who require hospitalization for unexplained pneumonia and who have an epidemiologic history that raises the suspicion of exposure, such as recent travel to a previously SARS-affected area (or close contact with an ill person with such a travel history), employment as a healthcare worker with direct patient contact or as a worker in a laboratory that contains live SARS-CoV, or an epidemiologic link to a cluster of cases of unexplained pneumonia. Once person-to-person SARS-CoV transmission has been documented anywhere in the world, the positive predictive value of even early clinical symptoms (e.g., fever or lower respiratory symptoms in the absence of pneumonia), while still low, may be sufficiently high — when combined with an epidemiologic link to settings in which SARS-CoV has been documented — to lead clinicians to consider a diagnosis of SARS-CoV disease.
In that context, the guidance that follows should be considered in the evaluation and management of patients who present from the community with fever or lower respiratory illnesses. For more detailed guidance on infection control, see Supplement I in Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
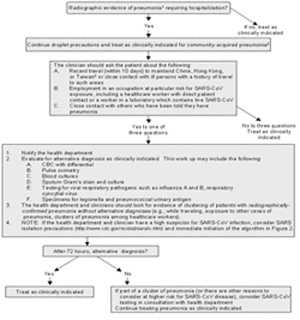
III. Guidelines for Evaluation of SARS-CoV Disease among Persons Presenting with Community-Acquired Illness (Figures 1 and 2)
The following is an approach for the evaluation of possible SARS-CoV disease among persons presenting with community-acquired illness. As part of the evaluation, in addition to identification of suggestive clinical features, clinicians should routinely incorporate into the medical history questions that may provide epidemiologic clues to identify patients with SARS-CoV disease.
Diagnosis of SARS-CoV Disease
In the absence of person-to-person transmission of SARS-CoV anywhere in the world, the diagnosis of SARS-CoV disease should be considered only in patients who require hospitalization for radiographically confirmed pneumonia and who have an epidemiologic history that raises the suspicion of SARS-CoV disease. The suspicion for SARS-CoV disease is raised if, within 10 days of symptom onset, the patient:
- Has a history of recent travel to mainland China, Hong Kong, or Taiwan (see Figure 1, footnote 3) or close contact1 with ill persons with a history of recent travel to such areas, or
- Is employed in an occupation at particular risk for SARS-CoV exposure, including a healthcare worker with direct patient contact or a worker in a laboratory that contains live SARS-CoV, or
- Is part of a cluster of cases of atypical pneumonia without an alternative diagnosis
Persons with such a clinical and exposure history should be evaluated according to the algorithm in Figure 1.
Once person-to-person transmission of SARS-CoV has been documented in the world, the diagnosis should still be considered in patients who require hospitalization for pneumonia and who have the epidemiologic history described above. In addition, all patients with fever or lower respiratory symptoms (e.g., cough, shortness of breath, difficulty breathing) should be questioned about whether within 10 days of symptom onset they have had
- Close contact with someone suspected of having SARS-CoV disease, OR
- A history of foreign travel (or close contact with an ill person with a history of travel) to a location with documented or suspected SARS-CoV, OR
- Exposure to a domestic location with documented or suspected SARS-CoV (including a laboratory that contains live SARS-CoV), or close contact with an ill person with such an exposure history.
Persons with such an exposure history should be evaluated for SARS-CoV disease according to the algorithm in Figure 2.
Footnote
- Close contact: A person who has cared for or lived with a person with SARS-CoV disease or had a high likelihood of direct contact with respiratory secretions and/or body fluids of a person with SARS-CoV disease. Examples of close contact include kissing or hugging, sharing eating or drinking utensils, talking within 3 feet, and direct touching. Close contact does not include activities such as walking by a person or briefly sitting across a waiting room or office.
IV. Additional Considerations
In some settings, early recognition of SARS-CoV disease may require additional measures. The following guidance is provided to assist in the evaluation of patients in settings or with characteristics not detailed/outlined in Figures 1 and 2. These include SARS outbreaks in the surrounding community, management of patients who become ill while already in the hospital, workers from laboratories that contain live SARS-CoV, pediatric patients, the elderly, and persons with chronic underlying diseases.
A. Additional epidemiologic risk factors to consider in community outbreak settings
The risk factors that should trigger suspicion for SARS-CoV disease may vary depending on the level of SARS-CoV transmission occurring in the community. Specifically, as outbreaks become more widespread, the types of epidemiologic characteristics that are considered as risk factors for SARS-CoV disease should be broadened appropriately. Two examples are given below.
1. Evaluating patients in the midst of a community outbreak in which more extensive secondary transmission of SARS-CoV is occurring in well-defined settings with all cases linked to other cases (e.g., an outbreak in a local hospital)
- Continue the activities for evaluation of persons with ‘fever and/or respiratory illness’ outlined in Figure 2, but in addition:
- Consider the diagnosis of SARS-CoV disease among all persons with radiographic evidence of pneumonia (even if not requiring hospitalization) if they:
- Have had exposure to hospitals in the 10 days before onset of symptoms (e.g., patient, visitor, or staff), or
- Are employed in an occupation at particular risk for SARS-CoV exposure, including a healthcare worker with or without direct patient contact or a worker in a clinical or research virology laboratory, or
- Have close contact with a patient with documented pneumonia.
2. Evaluating patients in the midst of a community outbreak in which transmission is widespread and epidemiologic linkages between cases are not well defined.
- Since epidemiologic links to persons with SARS-CoV disease may not be identifiable at this point, SARS-CoV disease should be considered in any patient presenting with fever or respiratory illness, even in the absence of known epidemiologic risk factors.
B. Management of patients who acquire illness while in the hospital
For persons with a high risk of exposure to SARS-CoV (e.g., persons previously identified through contact tracing as close contacts of a laboratory-confirmed case of SARS-CoV disease; persons who are epidemiologically linked to a laboratory-confirmed case of SARS-CoV disease), symptoms that should trigger the clinical algorithm should be expanded to include the presence of any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, diarrhea. For more details on the clinical features of SARS-CoV disease, see Figure 2, footnote 1.
C. Management of patients who acquire illness while in the hospital
This document focuses on the evaluation and management of patients who present from the community, although many of the same principles apply to hospitalized patients who develop nosocomial fever or lower respiratory symptoms. The diagnosis of nosocomial SARS-CoV disease may be particularly challenging, however, since many inpatients may have other reasons for developing nosocomial fever, lower respiratory symptoms, and pneumonia. Therefore, in hospitals known to have or suspected of having patients with SARS-CoV disease, clinicians and public health officials must be particularly vigilant about evaluating fever and respiratory illnesses among inpatients. Additional guidance on when to apply Figure 2 in the evaluation of patients who develop fever and/or respiratory illness while hospitalized is provided in Supplement C, Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
D. Laboratory workers
Breaks in technique in laboratories that contain live SARS-CoV can result in laboratory-acquired cases of SARS-CoV disease. Personnel working in laboratories that contain live SARS-CoV should report any febrile and/or lower respiratory illnesses to the supervisor, be evaluated for possible exposures, and be closely monitored for clinical features and course of illness. If laboratory workers with fever and/or lower respiratory illness are found to have an exposure to SARS-CoV, they should be managed according to the guidance in Figure 2. In addition, in an exposed laboratory worker, symptoms that should trigger the clinical algorithm in Figure 2 should be expanded to include the presence of any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, diarrhea (see Figure 2, footnote 1, for more information). Detailed information for persons who work in laboratories that contain live SARS-CoV is provided in Supplement F, Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
E. Considerations for the pediatric population
The document does not specifically address the evaluation and management of infants and children. Much less is known about SARS-CoV disease in pediatric patients than in adults. During the 2003 outbreaks, infants and children accounted for only a small percentage of patients and had much milder disease with better outcome. Their role in transmission is not well described but is likely much less significant than the role of adults. Taking these factors into account, the following guidance may change as more information becomes available on SARS-CoV disease in the pediatric population:
- In the absence of person-to-person SARS-CoV transmission in the world, evaluation and management for possible SARS-CoV disease should be considered only for adults, unless special circumstances make the clinician and health department consider a child to be of potentially high risk for having SARS-CoV disease.
- In the presence of person-to-person SARS-CoV transmission in the world, the evaluation algorithm established for adults can be used in children with the following caveats:
- Both the rate of development of radiographically confirmed pneumonia and the timing of development of such radiographic changes in children are unknown.
- The positive predictive value of rapid virus antigen detection tests (e.g., RSV) “in season” will be higher in a pediatric population.
- Pneumococcal and legionella urinary antigen testing are not recommended for routine diagnostic use in children.
F. Elderly persons and patients with underlying chronic illnesses
Typical symptoms of SARS-CoV disease may not always be present in elderly patients and those with underlying chronic illnesses, such as renal failure. Therefore, the diagnosis should be considered for almost any change in health status, even in the absence of typical clinical features of SARS-CoV disease, when such patients have epidemiologic risk factors for SARS-CoV disease (e.g., close contact with someone suspected to have SARS-CoV disease or exposure to a location [domestic or international] with documented or suspected recent transmission of SARS-CoV).
Figures
Top of Page
Related Pages
-
Core Document: App 1 - Clinical, Epidemiologic, and Virologic Features
Core section , Appendix 1 of Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS)
- Page last reviewed: May 3, 2005
- Page last updated: July 7, 2012
- Content source:


 ShareCompartir
ShareCompartir