Figure 2
Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness (version 2)
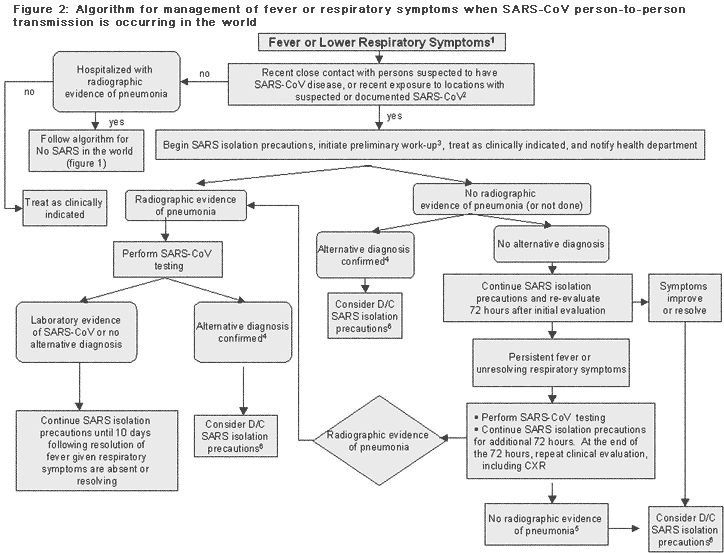
Text Version
Algorithm for management of fever or respiratory symptoms when SARS-CoV person-to-person transmission is occurring in the world
Flow chart elements as follows:
Fever or Lower Respiratory Symptoms1
Recent close contact with persons suspected to have SARS-CoV disease, or recent exposure to locations with suspected or documented SARS-CoV2
- if no...
- Hospitalized with radiographic evidence of pneumonia
- if no - treat as clinically indicated
- if yes - follow algorithm for No SARS in the world (figure 1)
- Hospitalized with radiographic evidence of pneumonia
- if yes...
Begin SARS isolation precautions, initiate preliminary work-up3, treat as clinically indicated, and notify health department
- if radiographic evidence of pneumonia perform SARS-CoV testing...
- if no radiographic evidence of pneumonia (or not done)...
- if alternative diagnosis confirmed4, consider D/C SARS isolation precautions6
- if no alternative diagnosis, continue SARS isolation precautions and re-evaluate 72 hours after initial evaluation
- if symptoms improve or resolve, consider D/C SARS isolation precautions6
- if persistent fever or unresolving respiratory symptoms, perform SARS-CoV testing, and continue SARS isolation precautions for additional 72 hours. At the end of the 72 hours, repeat clinical evaluation, including CXR
- if no radiographic evidence of pneumonia5, consider D/C SARS isolation precautions6
- if radiographic evidence of pneumonia return to step 1 above
Footnotes for Figure 2
- Clinical description of SARS-CoV disease and approach to treatment: Clinical judgment should be used to determine when symptoms trigger initiation of the algorithm in Figure 2. The early symptoms of SARS-CoV disease usually include fever, chills, rigors, myalgia, and headache. In some patients, myalgia and headache may precede the onset of fever by 12-24 hours. Respiratory symptoms often do not appear until 2-7 days after the onset of illness and most often include shortness of breath and/or dry cough. Diarrhea, sore throat, and rhinorrhea may also be early symptoms of SARS-CoV disease.
In the absence of fever, when screening patients for potential SARS-CoV disease, respiratory symptoms that would trigger the clinical algorithm are generally defined as lower respiratory tract symptoms (e.g., cough, shortness of breath, difficulty breathing). However, when screening patients who have a high risk of exposure to SARS-CoV (e.g., persons previously identified through contact tracing or self-identified as close contacts of a laboratory-confirmed case of SARS-CoV disease; persons who are epidemiologically linked to a laboratory-confirmed case of SARS-CoV disease), symptoms that should trigger the clinical algorithm should be expanded to include any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, diarrhea.
Although not diagnostic, the following laboratory abnormalities have been seen in some patients with laboratory-confirmed SARS-CoV disease:- Lymphopenia with normal or low white blood cell count
- Elevated hepatic transaminases
- Elevated creatine phosphokinase
- Elevated lactate dehydrogenase
- Elevated C-reactive protein
- Prolonged activated partial thromboplastin time
As of 1 December 2003, no specific treatment recommendations can be made for management of SARS-CoV disease. Empiric therapy for community-acquired pneumonia should include treatment for organisms associated with any community-acquired pneumonia of unclear etiology, including agents with activity against both typical and atypical respiratory pathogens. Treatment choices may be influenced by both the severity of and the circumstances surrounding the illness. Infectious disease consultation is recommended. The Infectious Diseases Society of America has guidelines for the management of community-acquired pneumonia. These guidelines can be found at online. - Exposure history for SARS-CoV, once SARS-CoV transmission is documented in the world:
In settings of no or limited local secondary transmission of SARS-CoV, patients are considered exposed to SARS if, within 10 days of symptom onset, the patient has:- Close contact with someone suspected of having SARS-CoV disease, OR
- A history of foreign travel (or close contact with an ill person with a history of travel) to a location with documented or suspected SARS-CoV, OR
- Exposure to a domestic location with documented or suspected SARS-CoV (including a laboratory that contains live SARS-CoV), or close contact with an ill person with such an exposure history.
- Clinical work-up: Clinicians should work up patients as clinically indicated. Depending on symptoms and exposure history, initial diagnostic testing for patients with suspected SARS-CoV disease may include:
- Complete blood count (CBC) with differential
- Chest radiograph
- Pulse oximetry
- Blood cultures
- Sputum Gram's stain and culture
- Testing for viral respiratory pathogens, notably influenza A and B and respiratory syncytial virus
- Legionella and pneumococcal urinary antigen testing if radiographic evidence of pneumonia (adults only)
SARS-CoV testing may be considered as part of the initial work-up if there is a high level of suspicion for SARS-CoV disease based on exposure history. For additional details on specialized laboratory testing options available through the health department and the Laboratory Response Network (LRN), see CDC's SARS website. - Alternative diagnosis:
An alternative diagnosis should be based only on laboratory tests with high positive-predictive value (e.g., blood culture, viral culture, Legionella urinary antigen, pleural fluid culture, transthoracic aspirate). In some settings, PCR testing for bacterial and viral pathogens can also be used to help establish alternative diagnoses. The presence of an alternative diagnosis does not necessarily rule out co-infection with SARS-CoV. - Radiographic testing:
Chest CT may show evidence of an infiltrate before a chest radiograph (CXR). Therefore, a chest CT should be considered in patients with a strong epidemiologic link to a known case of SARS-CoV disease and a negative CXR 6 days after onset of symptoms. Alternatively, the patient should remain in SARS isolation, and the CXR should be repeated on day 9 after symptom onset. - Discontinuation of SARS isolation precautions:
SARS isolation precautions should be discontinued only after consultation with the local public health authorities and the evaluating clinician. Factors that might be considered include the strength of the epidemiologic exposure to SARS-CoV, nature of contact with others in the residential or work setting, strength of evidence for an alternative diagnosis, and evidence for clustering of pneumonia among close contacts. Isolation precautions should be discontinued on the basis of an alternative diagnosis only when the following criteria are met:- Absence of strong epidemiologic link to known cases of SARS-CoV disease
- Alternative diagnosis confirmed using a test with a high positive-predictive value
- Clinical manifestations entirely explained by the alternative diagnosis
- No evidence of clustering of pneumonia cases among close contacts (unless >1 case in the cluster is confirmed to have the same alternative diagnosis)
- All cases of presumed SARS-CoV disease identified in the surrounding community can be epidemiologically linked to known cases or locations in which transmission is known to have occurred.
- Page last reviewed: May 3, 2005
- Page last updated: May 3, 2005
- Content source:


 ShareCompartir
ShareCompartir