Packing Diagnostic SARS Specimens for Transport: Summary Instructions
On This Page
Printer friendly version [3 pages]
NOTICE
Since 2004, there have not been any known cases of SARS reported anywhere in the world. The content in this Web site was developed for the 2003 SARS epidemic. But, some guidelines are still being used. Any new SARS updates will be posted on this Web site.
For more complete packing instructions see the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations and US DOT 49 CFR Parts 171-180
The U.S. Department of Transportation (DOT) Hazardous Materials (HazMat) Regulations, 49 CFR Parts 171-180, require all persons involved in the packaging, shipping, etc. of diagnostic specimens to be aware of the relevant regulations.
A Diagnostic Specimen is any human or animal material being transported for diagnostic or investigational purposes, BUT excluding live infected animals.
Diagnostic specimens transported under the IATA regulations are assigned to un identification number 3373.
Note: You, as the shipper – not the transport company – are responsible for the shipment until the package reaches the consignee.
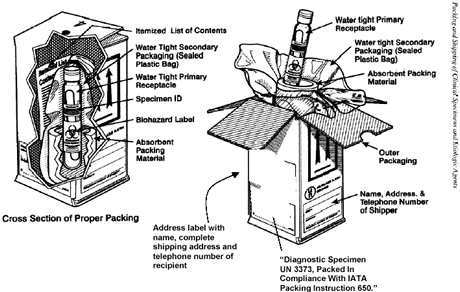
Primary Packaging
- Primary receptacle(s) must be water tight, e.g., if screw cap seal with parafilm or similar.
- Multiple primary receptacles must be wrapped individually to prevent breakage.
- When determining the volume of diagnostic specimens being shipped, include the viral transport media.
- Primary receptacle(s) must not contain more than 500 ml or 500 g.
the entire contents of the primary receptacle is the diagnostic specimen.
Secondary Packaging
- Use enough absorbent material to absorb the entire contents of all primary receptacles in case of leakage or damage.
- Secondary packaging must meet the IATA packaging requirements for diagnostic specimens including 1.2 meter (3.9 feet) drop test procedure. Since infectious substance packaging surpasses the requirements for diagnostic specimen packaging, in the IATA Packing Instruction 602, it can be used. Infectious substance packaging will have the required specification markings on packaging
- (“UN” will be in a circle): e.g., –

4G/CLASS 6.2/99/GB/2450
- (“UN” will be in a circle): e.g., –
- Secondary packaging must be watertight. Follow the packaging manufacturer or other authorized party’s packing instructions included with the secondary packaging.
- Secondary packaging must be at least 100 mm (4 inches) in the smallest overall external dimension.
- Must be large enough for shipping documents, e.g. air waybill.
Outer Packaging
- The outer packaging must not contain more than 4 L or 4 kg.
- Both dry ice and wet ice must be placed outside the secondary packaging.
- Dry ice: packaging must permit the release of carbon dioxide gas and not allow a build-up of pressure that could rupture the packaging.
- Wet ice: the packaging must be leak-proof.
- Each package and the air waybill must be marked with the following exact wording:
UN 3373 Diagnostic Specimen Packed in Compliance with IATA Packing Instruction 650
- An itemized list of contents must be enclosed between the secondary packaging and the outer packaging. Place in a sealed plastic bag to protect from moisture.
- A Shipper’s Declaration for Dangerous Goods is NOT required.
Proper packing and labeling of the secondary container for shipping of diagnostic specimens
- Page last reviewed: May 3, 2005
- Page last updated: January 25, 2013
- Content source:


 ShareCompartir
ShareCompartir