What is the Process of Technology Transfer?
CDC’s Technology Transfer Office is committed to transferring research into commercially viable products to address unmet public health needs. TTO accomplishes this goal by working as a liaison between CDC scientists and industry partners, to facilitate the technology transfer process. A simple description of this process along with the role of the TTO and CDC scientist is provided below.
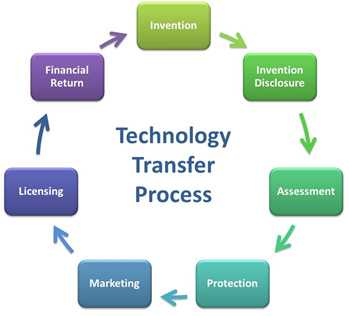
Invention
- What happens at this stage? The conception of an invention initiates the technology transfer process. The invention is typically conceived within the context of a research project. It can be a new technology or novel isolates collected during a surveillance study. At this stage, the invention is often in a raw form requiring additional development for it to become a commercial product. The conception of the invention may involve an individual or several individuals, sometimes at other institutions. The invention can also be the result of a collaborative research agreement.
- What is the role of the Inventor at this stage? It is important for the inventor to recognize when an invention has been developed or novel isolates have been collected. The inventor should maintain open communication with TTO to keep them informed of any developments. The inventor should also maintain detailed records on the development of the discovery and also note who was involved in the invention process. Contributing inventors are only the individuals involved in conceiving the invention. Individuals that simply reduce the invention to practice are not considered inventors.
- What is TTO doing at this stage? TTO is there to assist the CDC scientists during this initial phase by providing the scientists the advice and resources they need. Sometimes scientists may not realize they have a new discovery. TTO strives to help scientists at this step by actively engaging them and discussing their research to help them determine if they have developed a technology or material that is novel.
Invention Disclosure
- What happens at this stage? Once the invention has been conceived, inventors need to formally disclose it to the TTO. CDC scientists disclose technologies in an Employee Invention (and Discovery) Report form (EIR). The EIR form serves as the initial documentation of the invention. This form allows the inventor to clearly describe the innovation, its possible commercial applications, and details on who was involved in the invention process.
- What is the role of the Inventor at this stage? The cooperation of the inventor is extremely instrumental during this stage. Timing is everything during the technology transfer process, so inventors need to disclose the invention to TTO once it has been conceived to preserve its patentability. The inventor needs to fully disclose the invention, its improvements over current technologies, and the possible applications for the technology. To protect the patentability of the invention, CDC scientists are urged not to publically disclose or discuss the invention before consulting with TTO. If a public disclosure such as a publication or presentation has been made, the inventor should be prepared to provide the details of the disclosure.
- What is TTO doing at this stage? Not all CDC scientists are familiar with the disclosure process, so TTO assists inventors with the preparation of the EIR form. TTO educates inventors on the critical information they will need to provide as they prepare the EIR form. Since every invention differs, TTO contacts the inventor to discuss the details that are unique to their invention and situation.
Assessment
- What happens at this stage? When an EIR is received by TTO, the disclosure is evaluated to determine the marketability and patentability of the technology. TTO assesses the technology, discusses it with the scientist, and evaluates the potential of the technology. Based on the findings of this assessment, TTO makes recommendations on whether the technology should be patented. The recommendation, the EIR, and any pertinent information is then forwarded to the CDC Team at NIH. In some instances, TTO might recommend that a technology is not patented.
- What is the role of the Inventor at this stage? During the assessment stage, TTO needs the inventor to inform them of any changes or improvements to the technology. TTO also needs the inventor to be responsive. Several questions will arise during the assessment process regarding the invention. Since the inventor is the best source for information regarding the technology, the cooperation and attentiveness of the inventor is necessary.
- What is TTO doing at this stage? Upon completion of the EIR, a Technology Transfer Specialist is assigned the EIR for assessment. The Technology Transfer Specialists examines the technical details of the technology to determine what improvements it has over other products that are on the market. The Specialists will also consider other potential applications for the invention. TTO Specialists will search marketing and patent databases to provide a thorough assessment of the technology. These findings are drafted into a report. Once the report is prepared, a decision is made regarding the next steps for the technology. This recommendation is noted on the EIR form. The EIR form, the recommendation and any public disclosures are sent to the CDC Team at NIH for additional analysis.
Protection
- What happens at this stage? If the technology can be made into a commercial product that will potentially interest commercial partners, efforts are made to protect the invention. This protection is typically in the form of a US patent. Obtaining a patent can often be a lengthy process lasting 3 or more years. Foreign patent applications can also be filed in other countries to protect the invention abroad. This protection allows inventors and commercial partners to recover financial investments made to commercialize the product. The CDC Team at NIH works together with outside counsel in the patent filing and prosecution process for CDC technologies.
- What is the role of the Inventor at this stage? The inventor can expect to receive correspondence from TTO and the CDC Team at NIH regarding the patent protection process. The inventor needs to remain responsive throughout this stage. The inventor needs to ensure that if something has changed with the invention, it is communicated to the TTO. If a public disclosure has been made that can impact the patentability of the invention, the inventor will need to inform TTO promptly.
- What is TTO doing at this stage? Patent protection of CDC technologies involves a coordinated effort between TTO, the CDC Team at NIH, and external counsel that handles the patent filing and prosecution. This process is initiated when the TTO provides a recommendation to patent a technology that is sent to the NIH. The CDC Team at NIH reviews this recommendation, assesses the technology, and recommends a formal decision that is communicated to the TTO. The CDC Team at NIH staff then works with external counsel to draft, file, and prosecute the patent.
Marketing
- What happens at this stage? TTO markets available technologies to attract licensing opportunities. Sometimes this happens after a patent application has been filed on an invention. Marketing can also be initiated for research material that is not patentable but still offers some commercial value.
- What is the role of the Inventor at this stage? Inventors can often be the best source for information on potential commercial partners and unmet markets because they are often experts in their field. If the inventor is aware of commercial partners that have shown interest in their technology portfolio, the inventor should provide TTO their contact information for targeted marketing of technologies.
- What is TTO doing at this stage? TTO markets technologies available for licensing on CDC TTO’s website. Short non-confidential summaries are drafted for each CDC technology that is available for licensing. The summaries are used as marketing tools for potential licensing opportunities. These summaries are published on the NIH Office of Technology Transfer (OTT) website and are linked on the TTO website. CDC technologies are also marketed on the Federal Laboratory Consortium (FLC) Available Technologies page. A more expansive list of inventions are listed in CDC’s Available Technology Brochure that is used for marketing at conferences, meetings, and trade shows.
Licensing
- What happens at this stage? When a company is interested in securing rights to a CDC technology, a license is usually required. A license is an agreement where the owners of the invention give another party rights to the technology allowing them to produce, use, or sell it. The license defines the rights, responsibilities, exclusivity, and terms of the agreement.
- What is the role of the Inventor at this stage? Licenses are negotiated on behalf of the inventor by the CDC Team at NIH. Therefore, it is important that the inventor remains responsive to the TTO and NIH teams to ensure they are equipped with the information needed during their negotiations. Inventors should also communicate any questions or concerns regarding the license to the licensing manager that is handling their negotiations. If there are any technical developments or improvements to the technology that might impact the licensing process, the inventor should inform the licensing manager.
- What is TTO doing at this stage? The CDC Team at NIH negotiates the licensing of CDC technologies with potential commercial partners. Sometimes, licensing requests will be forwarded directly to TTO. In these cases, the Technology Transfer Specialists will assist the prospective licensee with the license application. The Specialist ensures that the license application is submitted to the CDC Team at NIH for further processing. For Biological Material License Agreements (BMLAs) where strains or isolates are being licensed out, the Specialists will assist the CDC scientists with the preparation of the EIR form for the strains.
Financial Return
- What happens at this stage? After a license is negotiated, post-license compliance must be maintained to ensure the scheduled development of the technology, payment of royalties, and compliance to the license agreement. Based on the terms negotiated in the agreement, a percentage of royalties will go to the inventor, while a portion will go to the originating laboratory for that technology. This allows funding to be reinvested into CDC for the development of additional technologies that can meet other public health needs.
- What is the role of the Inventor at this stage? The inventor should keep TTO informed of any changes in contact information to ensure that royalty payments are distributed appropriately.
- What is TTO doing at this stage? NIH and TTO team members coordinate efforts to ensure post-license compliance. When royalty payments are made to NIH, TTO staff members help to coordinate the receipt and distribution of the funding to the appropriate inventors and laboratories.
- Page last reviewed: March 30, 2017
- Page last updated: August 30, 2016
- Content source:
- Office of the Associate Director for Science


 ShareCompartir
ShareCompartir