Screening Recommendations and Considerations
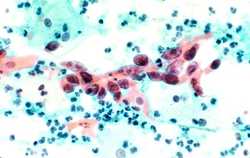
Cytological specimen showing cervical cancer, specifically squamous cell carcinoma in the cervix. Tissue is stained with pap stain and magnified x200. Source: NCI Visuals Online.
There are two cervical cancer screening tests—
- The cervical cytology (Pap test) looks for precancerous and cancerous cells.
- The high-risk HPV test looks for the DNA or RNA of the types of the HPV virus (16 and 18) that can cause these cellular changes.
Current Cervical Cancer Screening Recommendations
The U.S. Preventive Services Task Force (USPSTF) released cervical cancer screening recommendations with new screening intervals in March 2012. Annual screening is not recommended for average-risk women.
Screening Methods for Average-Risk Asymptomatic Women
- Age 21 to 29: Every 3 years with cytology (Pap testing), regardless of age of onset of sexual activity or other risk factors.
- Age 30 to 65: Every 5 years with HPV co-test (Pap + HPV test) OR every 3 years with cytology.
When NOT to Screen
- Younger Than Age 21: Screening is not recommended for women younger than age 21.
- Older Than Age 65: No screening past age 65 if adequate prior screening can be assessed accurately (three consecutive negative cytology results or two consecutive negative HPV results within 10 years before screening cessation, with the most recent test occurring within 5 years) and not otherwise at high risk for cervical cancer.
- No Cervix: No screening if the cervix was removed for a benign reason.
These screening recommendations do not apply to women—
- With a prior diagnosis of a high-grade precancerous cervical lesion or cervical cancer,
- With in utero exposure to diethylstilbestrol, or
- Who are immunocompromised (such as those who are HIV positive, organ transplant recipients, or on chronic corticosteroids).
For more details on each screening method, including performance characteristics, benefits, and harms, visit Cervical Cancer Screening–For Health Professionals.
Screening with the HPV Test Alone
In 2014, the Food and Drug Administration (FDA) approved the Roche HPV test for primary cervical cancer screening in women aged 25 and older. Shortly thereafter, the Society for Gynecologic Oncology published interim guidance [PDF-271KB] indicating that primary high-risk HPV screening can be considered as an alternative to current U.S. cytology-based cervical cancer screening methods. This guidance also recommends at least a 3-year screening interval after a negative primary HPV screening test. At this time, the USPSTF has not updated its recommendations to include primary HPV as an option for screening.
Knowledge Check
Answer the question on your own and check your answer.
If a 20-year-old sexually active woman requests a Pap test, what should you do? 
Answer: Educate her on screening recommendations, letting her know that Pap tests are not recommended for women under age 21, given that abnormal test results are likely to be transient and resolve on their own and resulting treatment may have adverse effects (for example, on future child-bearing). Offer HPV vaccination if she hasn’t received it yet or completed the series.
- Page last reviewed: September 16, 2015
- Page last updated: September 16, 2015
- Content source:


 ShareCompartir
ShareCompartir