Steps to Improve Lab Safety
Progress on laboratory safety
CDC laboratories routinely work with some of the most deadly germs in the world – identifying health threats and conducting vital public health research. CDC constantly develops and reviews extensive laboratory guidelines and procedures to protect both the public and laboratory workers. However, after a series of laboratory incidents, which posed no public risk, CDC investigated its laboratory practices and found areas for improvement. Although significant progress has been made since, CDC will continue to assess and improve its practices to protect the safety of CDC staff and the broader community.

Safety is the highest priority in CDC labs.
Background
In 2014, there were several unrelated incidents involving research samples of anthrax, influenza H5N1, and Ebola virus at CDC labs. Comprehensive internal and external reviews were conducted of each incident, and CDC created both internal and external workgroups to review the agency’s laboratory safety procedures and protocols, which resulted in recommendations for improving safety.
Steps taken to improve laboratory safety
CDC has begun multiple improvements to lab safety practices and procedures across the agency, with the goal of increasing CDC’s culture of safety. These ongoing efforts include:
- Establishing a new leadership position that will report to the CDC Director to provide agency-wide leadership and accountability for laboratory science, safety, and quality
- Continuing engagement with an external Laboratory Safety Workgroup to enhance best practices in laboratory safety and quality within CDC
- Implementing standardized disinfection practices across CDC’s infectious disease laboratories by developing new procedures for the use of validated and approved disinfectants
- Expanding biosafety training at CDC, including a complete review of the current biosafety training to revise and update current curriculum; held the first Biological Risk Assessment Course in February 2015, training laboratory staff on how to assess the risk associated with any laboratory science work
- Engaging with laboratory scientists on safety improvement measures: surveyed lab staff on safety concerns, hosted staff engagement sessions on lab safety, and held listening sessions with internal and external stakeholders to identify opportunities for safety improvements
- Revamping and implementing new and enhanced procedures for prompt reporting of laboratory incidents to CDC leadership and staff
- Conducting a search of approximately 1,000 rooms of labrelated space to ensure proper storage of select agents
- Completing a self-initiated, box and vial-by-by-vial inventory of more than 7 million samples in long-term storage for the infectious disease laboratories
- Rolling out a new electronic specimen inventory management system
- Enhancing procedures for custodianship of specimens with new entrance and exit processes for laboratory staff
- Convening a Laboratory Safety Review Board to conduct safety reviews of lab protocols for work in biosafety level 3 (BSL-3) and BSL-4 laboratories
- Installing cameras (80 to date) in laboratories as part of a “secondary verification” process to ensure that essential steps in a protocol are appropriately executed
- Establishing the Laboratory Leadership Service (LLS), a new fellowship program focused on growing managers in biosafety and laboratory processes; the first class of 7 fellows begins July 2015
- Expanding external accreditation for CDC laboratories to realize the full implementation of strong quality management systems across CDC
- Establishing a recognition program for CDC employees who demonstrate above-and-beyond efforts to improve laboratory safety
- Establishing ways to share lessons learned and promote transparency with the CDC laboratory community and with external partners

Working with dangerous germs is never without some risk.
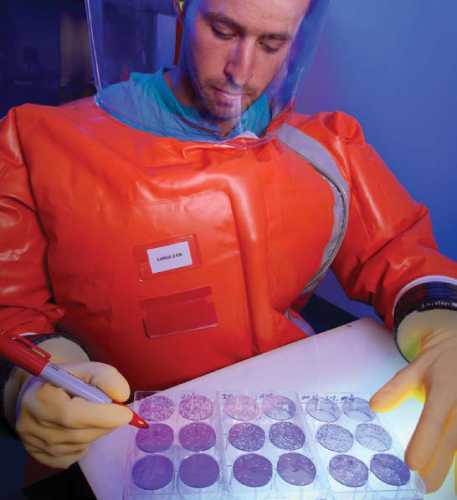
Laboratories are critical in fighting health threats.
Why laboratory safety is important
Laboratory safety cannot be achieved by a single set of standards or methods. Acceptable work practices in one lab may not be suitable for another lab and may expose workers to risk if used in another lab. Comprehensive biosafety practices are needed to allow scientists and researchers to work with specimens to identify new health threats, stop outbreaks, and gain new knowledge. CDC laboratories save lives and protect people, and CDC researchers are among the most dedicated scientists in the world. Laboratory work is critical, difficult, and can never be without risk. CDC scientists and staff remain dedicated to tracking and stopping any disease that threatens health, while also improving the culture of safety across the agency and minimizing risks to those who carry out this vitally important work.
To learn more about CDC’s 24/7 role in saving lives and protecting people visit About Us: https://www.cdc.gov/about/24-7/index.html
- Page last reviewed: August 5, 2015
- Page last updated: August 5, 2015
- Content source:


 ShareCompartir
ShareCompartir