The Vaccine Research Team
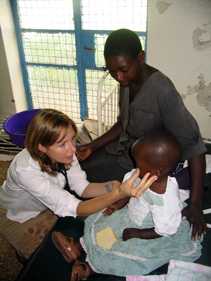
Dr. Mary Hamel is the principal investigator of the trial site in Siaya District in western Kenya. Formerly director of the Malaria Research Program at Kenya Medical Research Institute (KEMRI)/CDC (2005-2010), Dr. Hamel is the CDC Malaria Branch's senior technical advisor based in Washington, D.C.
Dr. Hamel and Dr. Simon Kariuki, KEMRI/CDC Malaria Branch Chief, lead the vaccine trial team at KEMRI/CDC. Other senior staff members of the KEMRI/CDC malaria vaccine research team are Chris Odero, site coordinator; Dr. Martina Oneko, lead pediatrician; Nobert Owino, field supervisor; Tony Sang, pharmacist; Maria Oziemkowskis, regulatory affairs; and Seth Oluoch, quality assurance. Dr. Laurence Slutsker, co–principal investigator, and Dr. Meredith McMorrow, pediatrician and medical epidemiologist, provide support from CDC Atlanta.
Site Coordinator Odero says, “Playing a role in the malaria vaccine trial has been the proudest moment of my life. I saw my cousin suffer from the debilitating effects of severe malaria and then die. Several other cousins also died of malaria. Because of the trial’s encouraging results and the presence of other effective interventions, I feel hopeful that my 8-month-old daughter will not suffer from malaria.”
To meet the demands of the vaccine trial, new clinical officers, nurses, medical officers, and other support staff were added at the district hospital’s pediatric ward. In all, more than 130 people are part of the malaria vaccine research team, including the hospital’s medical superintendent, who serves as a co-investigator, and many doctors, clinicians, and nurses. Already strong relationships were further reinforced, and all sick children who are admitted to the Siaya District Hospital pediatric ward during the trial will benefit from the additional expertise.
Preparations: Training, Equipment, Renovation
Many preparations were undertaken to meet the specific requirements for conducting a clinical trial, including these:
- training and supporting doctors and clinicians to provide medical care according to the comprehensive Kenyan Basic Pediatric Protocols and Basic Pediatric Life Support Guidelines;
- providing oxygen cylinders, pediatric monitors, and other clinical equipment required to provide care to severely ill children;
- building a laboratory with chemistry analyzers, blood culture machines, and other laboratory equipment, and providing the hospital with a new digital x-ray machine; and
- refurbishing the pediatric ward, repairing a leaking roof, fixing plumbing problems, and improving the acute care room, where the most severely ill children are stabilized.
- Page last reviewed: March 19, 2014
- Page last updated: March 19, 2014
- Content source:


 ShareCompartir
ShareCompartir