Tezepelumab
Tezepelumab (INN; development codes MEDI9929 and AMG 157) is a human monoclonal antibody designed for the treatment of asthma and atopic dermatitis.[2][3] It blocks thymic stromal lymphopoietin (TSLP), an epithelial cytokine that has been suggested to be critical in the initiation and persistence of airway inflammation.[4]
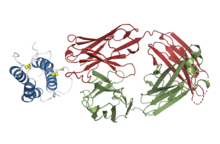 Structural basis for inhibition of TSLP-signaling by Tezepelumab (PDB 5J13)[1] | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | TSLP |
| Clinical data | |
| Other names | MEDI9929, AMG 157 |
| Routes of administration | Subcutaneous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C6400H9844N1732O1992S52 |
| Molar mass | 144590.40 g·mol−1 |
This drug is being developed in collaboration by MedImmune, LLC and Amgen.
It is in Phase III trials as of October 2018.
Structural studies by X-ray crystallography showed that Tezepelumab competes against a critical part of the TSLPR binding site on TSLP[1]
References
- Verstraete, Kenneth; Peelman, Frank; Braun, Harald; Lopez, Juan; Van Rompaey, Dries; Dansercoer, Ann; Vandenberghe, Isabel; Pauwels, Kris; Tavernier, Jan (April 2017). "Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma". Nature Communications. 8 (1): 14937. doi:10.1038/ncomms14937. ISSN 2041-1723. PMC 5382266. PMID 28368013.
- Statement On A Nonproprietary Name Adopted By The USAN Council - Tezepelumab, American Medical Association.
- World Health Organization (2015). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 113" (PDF). WHO Drug Information. 29 (2).
- Tezepelumab granted Breakthrough Therapy Designation by US FDA - AstraZeneca Press Release
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.