Side effects of bicalutamide
The side effects of bicalutamide, a nonsteroidal antiandrogen (NSAA), including its frequent and rare side effects, have been well-studied and characterized. The most common side effects of bicalutamide monotherapy in men include breast tenderness, gynecomastia, feminization, demasculinization, and hot flashes. Less common side effects of bicalutamide monotherapy in men include sexual dysfunction, depression, fatigue, weakness, and anemia. Bicalutamide is well-tolerated and has few side effects in women. General side effects of bicalutamide that may occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.
| Frequency | Class | Side effect |
|---|---|---|
| Very common (≥10%) | Reproductive system and breast disorders | • Breast tendernessa • Gynecomastiaa |
| Common (≥1% and <10%) | General and psychiatric disorders | • Asthenia • Decreased libido • Erectile dysfunction • Hot flashes |
| Skin and subcutaneous tissue disorders | • Decreased body hair | |
| Hepato-biliary disorders | • Elevated liver enzymesb | |
| Uncommon (≥0.1% and <1%) | Immune system disorders | • Hypersensitivity reactions, including angioedema and hives |
| Rare (<0.1%) and unknown | Respiratory, thoracic, and mediastinal disorders | • Interstitial lung diseasec |
| Skin and subcutaneous tissue disorders | • Sensitivity to light | |
| Hepato-biliary disorders | • Liver toxicityd | |
Footnotes and sources
a = Incidence of breast changes of up to 80 to 90%. Mild-to-moderate in 90% of cases. Incidence greatly decreased in combination with castration. b = Elevated liver enzymes rarely severe and usually transient, resolving or improving with continued therapy or with discontinuation. Incidence of 3.4% relative to 1.9% for placebo in a high-dose (150 mg/day) 4,000-patient trial. c = Six case reports of interstitial lung disease published (as of 2016). Incidence of 0.01% (12 patients) in an 87,000-patient cohort. d = Seven case reports of hepatotoxicity published (as of 2018). No cases in a high-dose (150 mg/day) 4,000-patient trial (suggesting incidence of <0.03%). Sources: [1][2][3][4][5][6][7] | ||
In men with prostate cancer, bicalutamide monotherapy has been associated with an increased risk of non-cancer death, in part due to an increased incidence of heart failure. This is thought to be a consequence of androgen deprivation. Bicalutamide monotherapy has been found to cause unfavorable liver changes in around 3% of men, with such changes necessitating discontinuation in about 0.3 to 1% of men. Very rarely, bicalutamide has been associated with liver damage, lung disease, and sensitivity to light. It has also uncommonly been associated with hypersensitivity reactions. Bicalutamide has a theoretical risk of birth defects in male fetuses.
| Side effect | Bicalutamide 150 mg/day + standard care (n = 4022) (%)a,b | Placebo + standard care (n = 4031) (%)a,b |
|---|---|---|
| Gynecomastiac | 66.2 | 7.7 |
| With breast pain | 53.1 | ND |
| Without breast pain | 13.1 | ND |
| Breast painc | 72.8 | 7.2 |
| With gynecomastia | 53.1 | ND |
| Without gynecomastia | 19.7 | ND |
| Asthenia | 10.2 | 7.2 |
| Pharyngitis | 10.0 | 10.5 |
| Erectile dysfunction | 9.0 | 6.1 |
| Hot flashesc | 9.0 | 5.2 |
| Back pain | 8.7 | 10.4 |
| Constipation | 8.2 | 6.7 |
| Arthralgia | 7.3 | 8.9 |
| Urinary tract infection | 7.1 | 6.1 |
| Flu-like symptoms | 7.0 | 7.1 |
| Abdominal pain | 6.6 | 6.6 |
| Diarrhea | 6.3 | 6.4 |
| Hypertension | 6.2 | 6.5 |
| Pelvic pain | 6.2 | 6.2 |
| Pain | 6.1 | 6.7 |
| Rash | 6.1 | 6.3 |
| Urinary incontinence | 6.0 | 5.7 |
| Decreased hair growth | 5.9 | 0.8 |
| Weight gain | 5.7 | 2.8 |
| Urinary tract disorder | 5.4 | 6.6 |
| Peripheral edema | 5.1 | 4.4 |
| Hernia | 4.7 | 5.6 |
| Hematuria | 4.1 | 5.5 |
| Accidental injury | 4.0 | 5.1 |
| Decreased libido | 3.6 | 1.1 |
| Abnormal liver function testsd | 3.4 | 1.9 |
| Footnotes: a = In the Early Prostate Cancer (EPC) trial at 5.4-year follow-up in men with early prostate cancer (localized or locally advanced prostate cancer). b = Incidence >5% regardless of causality, with a few additional side effects of relevance of <5% incidence. c = Mild-to-moderate in >90% of cases. d = Mainly transient and rarely severe. Sources: [1][8][9] | ||
| Side effecta,b | 10 mg/day (n = 45) | 30 mg/day (n = 75) | 50 mg/day (n = 147) | 100 mg/day (n = 56) | 150 mg/day (n = 66) | 200 mg/day (n = 29) |
|---|---|---|---|---|---|---|
| Pharmacological (antiandrogenic) side effects | ||||||
| Gynecomastia | 4/44 (9%) | 14/54 (26%) | 50/137 (36%) | 44/56 (79%) | 50/64 (78%) | 23/29 (79%) |
| Breast tenderness | 5/44 (11%) | 22/53 (42%) | 67/139 (48%) | 48/56 (86%) | 57/64 (89%) | 23/29 (79%) |
| Hot flashes | 7/42 (17%) | 5/51 (10%) | 17/132 (13%) | 9/52 (17%) | 13/61 (21%) | 11/27 (41%) |
| Decreased libido | 6/14 (43%) | 5/9 (56%) | 16/35 (46%) | 8/10 (80%) | 11/16 (69%) | 4/8 (50%) |
| Decreased potency | 4/11 (36%) | 6/9 (67%) | 9/24 (38%) | 7/8 (88%) | 7/12 (58%) | 4/7 (57%) |
| Non-pharmacological side effects | ||||||
| Anemia | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5.4%) | 4 (6.1%) | 0 (0%) |
| Asthenia | 5 (11.1%) | 4 (5.3%) | 13 (8.8%) | 3 (5.4%) | 0 (0%) | 0 (0%) |
| Back pain | 3 (6.7%) | 4 (5.3%) | 29 (19.7%) | 8 (14.3%) | 8 (12.1%) | 2 (6.9%) |
| Bone pain | 7 (15.6%) | 13 (17.3%) | 19 (12.9%) | 8 (14.3%) | 0 (0%) | 0 (0%) |
| Constipation | 3 (6.7%) | 0 (0%) | 12 (8.2%) | 7 (12.5%) | 7 (10.6%) | 3 (10.3%) |
| Diarrhea | 0 (0%) | 0 (0%) | 8 (5.4%) | 3 (5.4%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (6.9%) |
| Dyspnea | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (6.1%) | 0 (0%) |
| Dysuria | 0 (0%) | 0 (0%) | 19 (12.9%) | 9 (16.1%) | 4 (6.1%) | 2 (6.9%) |
| Hematuria | 0 (0%) | 0 (0%) | 9 (6.1%) | 4 (7.1%) | 6 (9.1%) | 2 (6.9%) |
| Malaise | 0 (0%) | 4 (5.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 3 (6.7%) | 8 (10.7%) | 8 (5.4%) | 0 (0%) | 4 (6.1%) | 0 (0%) |
| Pain | 7 (15.6%) | 4 (5.3%) | 19 (12.9%) | 8 (14.3%) | 12 (18.2%) | 5 (17.2%) |
| Pelvic pain | 4 (8.9%) | 5 (6.7%) | 16 (10.9%) | 8 (14.3%) | 6 (9.1%) | 2 (6.9%) |
| Pruritus | 0 (0%) | 4 (5.3%) | 8 (5.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rash | 0 (0%) | 4 (5.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Somnolence | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7.6%) | 2 (6.9%) |
| Urinary frequency | 5 (11.1%) | 0 (0%) | 13 (8.8%) | 12 (21.4%) | 13 (19.7%) | 0 (0%) |
| Urinary incontinence | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (6.9%) |
| Urinary retention | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5.4%) | 4 (6.1%) | 0 (0%) |
| Urinary tract infection | 0 (0%) | 0 (0%) | 8 (5.4%) | 5 (8.9%) | 12 (18.2%) | 0 (0%) |
| Urinary urgency | 8 (17.8%) | 9 (12.0%) | 22 (15.0%) | 0 (0%) | 6 (9.1%) | 2 (6.9%) |
| Urination impaired | 5 (11.1%) | 8 (10.7%) | 21 (14.3%) | 7 (12.5%) | 5 (7.6%) | 0 (0%) |
| Vomiting | 3 (6.7%) | 7 (9.3%) | 10 (6.8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Weight gain | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (12.1%) | 3 (10.3%) |
| ALT increased | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (6.1%) | 0 (0%) |
| AST increased | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (13.6%) | 0 (0%) |
| γ-GT increased | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (6.1%) | 0 (0%) |
| Color key: Black: <5%; Blue: 5–10%; Green: 10–25%; Orange: 25–50%; Red: >50%. Footnotes: a = In three phase II studies of 390 men with advanced prostate cancer treated for a minimum of 12 weeks. b = Incidence >5%, regardless of causality. Sources: [10] | ||||||
Breast changes
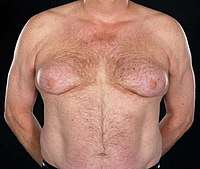
The most common side effects of bicalutamide monotherapy in men are breast pain/tenderness and gynecomastia.[15] These side effects may occur in as many as 90% of men treated with bicalutamide monotherapy,[16] but gynecomastia is generally reported to occur in 70 to 80% of patients.[17] In the EPC trial, at a median follow-up of 7.4 years, breast pain and gynecomastia respectively occurred in 73.6% and 68.8% of men treated with 150 mg/day bicalutamide monotherapy.[18][19] Gynecomastia associated with NSAA monotherapy usually develops within the first 6 to 9 months following initiation of treatment.[14] In more than 90% of affected men, bicalutamide-related breast changes are mild-to-moderate in severity.[19][20] It is only rarely and in severe and extreme cases of gynecomastia that the proportions of the male breasts become so marked that they are comparable to those of women.[21] In addition, bicalutamide-associated breast changes improve or resolve in most men upon discontinuation of therapy.[19] In the EPC trial, 16.8% of bicalutamide patients relative to 0.7% of controls withdrew from the study due to breast pain and/or gynecomastia.[20] The incidence and severity of gynecomastia are reportedly higher with estrogens (e.g., diethylstilbestrol) than with NSAAs like bicalutamide in the treatment of men with prostate cancer.[22]
Management
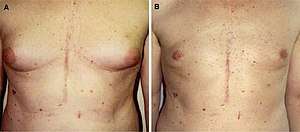
Tamoxifen, a selective estrogen receptor modulator (SERM) with antiestrogenic actions in breast tissue and estrogenic actions in bone, has been found to be highly effective in preventing and reversing bicalutamide-induced gynecomastia in men.[24][25] Moreover, in contrast to GnRH analogues (which also alleviate bicalutamide-induced gynecomastia), tamoxifen poses minimal risk of accelerated bone loss and osteoporosis.[24][25] For reasons that are unclear, anastrozole, an aromatase inhibitor (or an inhibitor of estrogen biosynthesis), has been found to be much less effective in comparison to tamoxifen for treating bicalutamide-induced gynecomastia.[24][25] A systematic review of NSAA-induced gynecomastia and breast tenderness concluded that tamoxifen (10–20 mg/day) and radiotherapy could effectively manage the side effect without relevant adverse effects, though with tamoxifen showing superior effectiveness.[26] Surgical breast reduction may also be employed to correct bicalutamide-induced gynecomastia.[27]
| Time | Placebo | 1 mg/day | 2.5 mg/day | 5 mg/day | 10 mg/day | 20 mg/day |
|---|---|---|---|---|---|---|
| 6 months | 98% | 90% | 80% | 54% | 22% | 10% |
| 12 months | 99% | 95% | 84% | 56% | 38% | 19% |
| Notes: Prevention of bicalutamide-induced breast symptoms with tamoxifen in men. Breast symptoms were specifically gynecomastia and breast pain. Sources: [28][29] | ||||||
Sexual dysfunction
Bicalutamide may cause sexual dysfunction, including decreased sex drive and erectile dysfunction.[18] However, the rates of these side effects with bicalutamide monotherapy are very low.[18] In the EPC trial, at 7.4 years follow-up, the rates of decreased libido and impotence were only 3.6% and 9.3% in the 150 mg/day bicalutamide monotherapy group relative to 1.2% and 6.5% for placebo, respectively.[18] Similarly, in the trials of 150 mg/day bicalutamide monotherapy for advanced prostate cancer, fewer than 10% of men reported decreased sex drive or reduced erectile function as a side effect.[19] About two-thirds of men in these trials, who had advanced prostate cancer and were of almost invariably advanced age,[30] maintained sexual interest, while sexual function was slightly reduced by 18%.[19] Most men experience sexual dysfunction only moderately or not at all with bicalutamide monotherapy, and the same is true during monotherapy with other NSAAs.[15] Bicalutamide monotherapy at a dosage of 50 mg/day had no effect on nocturnal erections in men with prostate cancer.[31][32]
Similarly to in men, bicalutamide has been associated with minimal or no sexual dysfunction in women.[33] A phase III clinical study of 50 mg/day bicalutamide in conjunction with a combined oral contraceptive in women with severe hirsutism due to polycystic ovary syndrome (PCOS) carefully assessed the side effect of decreased libido and found that the incidence with bicalutamide did not differ from the control group.[33] Minimal rates of reduced sex drive have also been associated with the related NSAA flutamide.[34][35] These findings are in accordance with the fact that women with complete androgen insensitivity syndrome (CAIS) show normal sexual function in spite of complete loss of androgen receptor (AR) signaling,[36] as well as with a variety of findings concerning testosterone levels and sexual function in premenopausal women in which no change in parameters of sexual function including libido have been observed in association with increases or decreases in testosterone levels.[36] It appears that testosterone levels within the normal physiological range are not importantly involved in sexual desire or function in women.[37]
Reproductive changes
Bicalutamide reduces the size of the prostate gland and seminal vesicles,[38] though not of the testes.[39] Significantly reduced penile length is also a recognized adverse effect of ADT.[40][41] Reversible hypospermia or aspermia (that is, reduced or absent semen/ejaculate production) may occur.[42][43] However, bicalutamide does not appear to adversely affect spermatogenesis, and thus may not necessarily abolish the capacity/potential for fertility in men.[39][44] Due to the induction of chronic overproduction of LH and testosterone, there was concern that long-term bicalutamide monotherapy might induce Leydig cell hyperplasia and tumors (usually benign),[45] but clinical studies indicate that Leydig cell hyperplasia does not occur to a clinically important extent.[46][44][47]
Gastrointestinal
The incidence of diarrhea with bicalutamide monotherapy in the EPC trial was comparable to placebo (6.3% vs. 6.4%, respectively).[19] In phase III studies of bicalutamide monotherapy for LAPC, the rates of diarrhea for bicalutamide and castration were 6.4% and 12.5%, respectively, the rates of constipation were 13.7% and 14.4%, respectively, and the rates of abdominal pain were 10.5% and 5.6%, respectively.[48]
Hot flashes
In the EPC trial, at 7.4 years follow-up, the rate of hot flashes was 9.2% for bicalutamide monotherapy relative to 5.4% for placebo, which was regarded as relatively low.[18] In the LAPC subgroup of the EPC trial, the rate of hot flashes with bicalutamide monotherapy was 13.1% (relative to 50.0% for castration).[18][19]
Psychological
At 5.3 years follow-up, the incidence of depression was 5.5% for bicalutamide monotherapy relative to 3.0% for placebo in the EPC trial, and the incidence of asthenia (weakness or fatigue) was 10.2% for bicalutamide monotherapy relative to 5.1% for placebo.[49]
Anemia
Androgens are known to stimulate the formation of red blood cells and increase the red blood cell count and circulating hematocrit levels, effects which they mediate by increasing production of erythropoietin in the kidneys.[50] In accordance, anabolic–androgenic steroids (AAS) such as oxymetholone and nandrolone decanoate are approved and used in the treatment of severe anemia,[50][51] and AAS can cause polycythemia as an adverse effect in high dosages.[50] Conversely, whether via castration, NSAA monotherapy, or CAB, mild anemia is a common side effect of ADT in men.[46][52] The incidence of anemia with bicalutamide as a monotherapy or with castration was about 7.4% in clinical trials.[46] A decrease of hemoglobin levels of 1–2 g/dL after approximately six months of treatment may be observed.[52]
Skin changes
Androgens are involved in regulation of the skin (e.g., sebum production), and antiandrogens are known to be associated with skin changes.[46] Skin-related side effects, which included dry skin, itching, and rash, were reported at a rate of 2% in both monotherapy and CAB clinical studies of bicalutamide in men.[46]
With castration
Combination of bicalutamide with medical (i.e., a GnRH analogue) or surgical castration modifies the side effect profile of bicalutamide. Some of its side effects, including breast pain/tenderness and gynecomastia, are far less likely to occur when the drug is combined with a GnRH analogue,[53] while certain other side effects, including hot flashes, depression, fatigue, and sexual dysfunction,[54] occur much more frequently in combination with a GnRH analogue.[31][55][56] It is thought that this is due to the suppression of estrogen levels (in addition to androgen levels) by GnRH analogues, as estrogens may compensate for various negative central effects of androgen deprivation.[31] If bicalutamide is combined with a GnRH analogue or surgical castration, the elevation of androgen and estrogen levels in men caused by bicalutamide will be prevented and the side effects of excessive estrogens, namely gynecomastia, will be reduced.[53] However, due to the loss of estrogen, bone loss will accelerate and the risk of osteoporosis developing with long-term therapy will increase.[57]
Increased mortality
In the LPC group of the EPC study, although 150 mg/day bicalutamide monotherapy had reduced mortality due to prostate cancer relative to placebo, there was a trend toward significantly increased overall mortality for bicalutamide relative to placebo at 5.4-year follow-up (25.2% vs. 20.5%).[49][58][59] This was because more bicalutamide than placebo recipients had died due to causes unrelated to prostate cancer in this group (16.8% vs. 9.5% at 5.4-year follow-up; 10.2% vs. 9.2% at 7.4-year follow-up).[49][59][18] At 7.4-year follow-up, there were numerically more deaths from heart failure (1.2% vs. 0.6%; 49 vs. 25 patients) and gastrointestinal cancer (1.3% vs. 0.9%) in the bicalutamide group relative to placebo recipients, although cardiovascular morbidity was similar between the two groups and there was no consistent pattern suggestive of drug-related toxicity for bicalutamide.[18][60] In any case, although the reason for the increased overall mortality with 150 mg/day bicalutamide monotherapy has not been fully elucidated,[17] it has been said that the finding that heart failure was twice as frequent in the bicalutamide group warrants further investigation.[61] In this regard, it is notable that low testosterone levels in men have been associated in epidemiological studies with cardiovascular disease as well as with a variety of other disease states (including hypertension, hypercholesterolemia, diabetes, obesity, Alzheimer's disease, osteoporosis, and frailty).[62]
According to Iversen et al. (2006), the increased non-prostate cancer mortality with bicalutamide monotherapy in LPC patients has also been seen with castration (via orchiectomy or GnRH analogue monotherapy) and is likely a consequence of androgen deprivation in men rather than a specific drug toxicity of bicalutamide:[63]
The increased number of deaths in patients with localized disease receiving bicalutamide was meticulously investigated and they appeared to be due to a number of small imbalances rather than a specific cause. In addition, no direct toxic effect on any organ system could be identified. From this it may be speculated that the excess deaths in patients who are at low risk from prostate cancer mortality reflect the impact of endocrine therapy (rather than bicalutamide in particular). [...] The increased number of non-prostate cancer deaths in the early castration therapy arm [(via orchiectomy or GnRH monotherapy)] in the [Medical Research Council] study suggests that the trend towards an increased number of deaths in patients with localized disease in the present study is a reflection of early endocrine therapy as a concept rather than a bicalutamide-related phenomenon.[63]
A study of 300 to 600 mg/day bicalutamide monotherapy in 248 men with LAPC or metastatic prostate cancer found that there were no effects of bicalutamide on heart rate, blood pressure, or electrocardiogram parameters.[64][65] In addition, at 5-year follow-up, the incidence of cardiovascular events was low, with no differences between the bicalutamide and castration groups.[64][65] There were also no differences in the incidences of arrhythmia, myocardial infarction, or other ischemic cardiac or cerebrovascular conditions.[64][65] These findings suggest that bicalutamide does not cause an excess in cardiovascular events or conditions.[64][65]
A meta-analysis of prospective randomized clinical trials of GnRH agonist-based ADT for the treatment of non-metastatic prostate cancer that included over 4,000 patients found no evidence of increased cardiovascular mortality or overall mortality.[66] Non-prostate cancer mortality was not specifically assessed.[66]
A case report in which bicalutamide was described as a probable cause of heart failure in an elderly man with prostate cancer has been published.[67]
Renal toxicity
As a result of androgen deprivation therapy, bicalutamide may increase the risk of kidney failure in men.[68]
Liver toxicity
Bicalutamide may cause liver changes rarely, such as elevated transaminases and jaundice.[69] In the EPC study of 4,052 prostate cancer patients who received 150 mg/day bicalutamide as a monotherapy, the incidence of abnormal liver function tests was 3.4% for bicalutamide and 1.9% for standard care (a 1.5% difference potentially attributable to bicalutamide) at 3-year median follow-up.[18][70] For comparison, the incidences of abnormal liver function tests are 42 to 62% for flutamide, 2 to 3% for nilutamide,[69][71] and (dose-dependently) between 10% and 28% for CPA,[72][73][74] whereas there appears to be no risk with enzalutamide.[75][76] In the EPC trial, bicalutamide-induced liver changes were usually transient and rarely severe.[18] The medication was discontinued due to liver changes (manifested as hepatitis or marked increases in liver enzymes) in approximately 0.3% to 1% of patients treated with it for prostate cancer in clinical trials.[77][78]
The risk of liver changes with bicalutamide is considered to be small but significant, and monitoring of liver function is recommended.[18][79] Elevation of transaminases above twice the normal range or jaundice may be an indication that bicalutamide should be discontinued.[80] Liver changes with bicalutamide usually occur within the first 3 or 4 months of treatment, and it is recommended that liver function be monitored regularly for the first 4 months of treatment and periodically thereafter.[77] Symptoms that may indicate liver dysfunction include nausea, vomiting, abdominal pain, fatigue, anorexia, "flu-like" symptoms, dark urine, and jaundice.[77]
A total of 7 case reports of bicalutamide-associated hepatotoxicity or liver failure, two of which were fatal, have been published in the literature as of 2018.[81][69][82] One of these cases occurred after two doses of bicalutamide, and has been said to more likely to have been caused by prolonged prior exposure of the patient to flutamide and CPA.[69][71][83][84][85] In the reported cases of bicalutamide-associated hepatotoxicity, the dosages of the drug were 50 mg/day (three), 80 mg/day (one), 100 mg/day (one), and 150 mg/day (two).[81][82] Relative to flutamide (which has an estimated incidence rate of 0.03% or 3 per 10,000), hepatotoxicity is far rarer with bicalutamide and nilutamide, and bicalutamide is regarded as having the lowest risk of the three medications.[86][83][87] For comparison, by 1996, 46 cases of severe cholestatic hepatitis associated with flutamide had been reported, with 20 of the cases resulting in death.[72] Moreover, a 2002 review reported that there were 18 reports of hepatotoxicity associated with CPA in the medical literature, with 6 of the reported cases resulting in death, and the review also cited a report of an additional 96 instances of hepatotoxicity that were attributed to CPA, 33 of which resulted in death.[72]
The clinical studies that have found elevated liver enzymes and the case reports of hepatotoxicity with bicalutamide have all specifically pertained to men of advanced age with prostate cancer.[18][70][69][82] It is notable that older age, for a variety of reasons, appears to be an important risk factor for drug-induced hepatotoxicity.[88][89] As such, the risk of liver changes with bicalutamide may be less in younger patients, for instance young hirsute women and transgender women.[88][89] However, it has been reported on the basis of very limited evidence that this may not be the case with flutamide.[90]
From a theoretical standpoint (on the basis of structure–activity relationships), it has been suggested that flutamide, bicalutamide, and nilutamide, to varying extents, all have the potential to cause liver toxicity.[91] However, in contrast to flutamide, hydroxyflutamide, and nilutamide, bicalutamide exhibits much less or no mitochondrial toxicity and inhibition of enzymes in the electron transport chain such as respiratory complex I (NADH ubiquinone oxidoreductase), and this may be the reason for its much lower risk of hepatotoxicity in comparison.[92][93][94][95] The activity difference may be related to the fact that flutamide, hydroxyflutamide, and nilutamide all possess a nitroaromatic group, whereas in bicalutamide, a cyano group is present in place of this nitro group, potentially reducing toxicity.[83][92][95][96]
| # | Age | Sex | Dosage | Onset | Type of injury | Outcome | Ref | |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 years | Male | 50 mg/day | 2 days | Hepatocellular | Survived | Dawson et al. (1997) | |
| 2 | 59 years | Male | ?a | 4 days | Hepatocellular | Death | O'Bryant et al. (2008) | |
| 3 | 61 years | Male | 50 mg/day | ~106 days | ? | Death | Castro Beza et al. (2008) | |
| 4 | 81 years | Male | 150 mg/day | 21 days | Mixed | Survived | Hussain et al. (2014) | |
| 5 | 62 years | Male | 100 mg/day | 133 days | Hepatocellular | Survived | Yun et al. (2016) | |
| 6 | 67 years | Male | 150 mg/day | 23 days | Mixed | Survived | Gretarsdottir et al. (2018) | |
| 7 | 74 years | Male | 80 mg/day | 42 days | ? | Survived | Kotoh et al. (2018) | |
| Footnotes: a = Likely 50 mg/day. Notes: Additional cases of bicalutamide-associated adverse liver changes have been reported; these include 11 cases in a 2006 Spanish pharmacovigilance system report (including 1 case of hepatitis, 2 cases of cholestatic hepatitis, 1 case of jaundice, 4 cases of elevated liver enzymes, and 1 case of elevated bilirubin; no deaths) and a number of cases in the FDA Adverse Event Reporting System (FAERS). Sources: Main: [81][97] | ||||||||
Lung toxicity
Several case reports of interstitial pneumonitis (which can progress to pulmonary fibrosis) in association with bicalutamide treatment have been published in the medical literature.[98][99][100][101] Interstitial pneumonitis with bicalutamide is said to be an extremely rare event,[102] and the risk is far less relative to that seen with nilutamide (which has an incidence rate of 0.5–2% of patients).[103]:81[99][104] In a very large cohort of prostate cancer patients, the incidence of interstitial pneumonitis with NSAAs was 0.77% for nilutamide but only 0.04% (4 per 10,000) for flutamide and 0.01% (1 per 10,000) for bicalutamide.[3] An assessment done prior to the publication of the aforementioned study estimated the rates of pulmonary toxicity with flutamide, bicalutamide, and nilutamide as 1 case, 5 cases, and 303 cases per million, respectively.[105] In addition to interstitial pneumonitis, a single case report of eosinophilic lung disease in association with six months of 200 mg/day bicalutamide treatment exists.[106][107] Side effects associated with the rare potential pulmonary adverse reactions of bicalutamide may include dyspnea (difficult breathing or shortness of breath), cough, and pharyngitis (inflammation of the pharynx, resulting in sore throat).[108]
| # | Age | Sex | Dosage | Onset | Type of injury | Outcome | Ref | |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 years | Male | 200 mg/day | 6 months | Eosinophilic lung disease | Recovered | Wong et al. (1998) | |
| 2 | ~76 years | Male | 200 mg/day | 8 months | Interstitial pneumonitis | Recovered | McCaffrey & Scher (1998) | |
| 3 | ~82 years | Male | 80 mg/day | 4 weeks | Interstitial pneumonitis | Recovered | Shioi et al. (2003) | |
| 4 | ~72 years | Male | 80 mg/day | 2.5 months | Interstitial pneumonitis | Recovered, then deatha | Shioi et al. (2005) | |
| 5 | 84 years | Male | ? mg/day | ? | Interstitial pneumonitis | Recovered | Kobayashi et al. (2006) | |
| 6 | 78 years | Male | 80 mg/day | 8 months | Interstitial pneumonitis | Recovered | Masago et al. (2011) | |
| 7 | 77 years | Male | ? mg/day | 7 months | Interstitial pneumonitis | Death | Song et al. (2014) | |
| 8 | 77 years | Male | >50 mg/day | ~12 months | Interstitial pneumonitis | Death | Molina Mancero et al. (2016) | |
| 9 | 66 years | Male | ? mg/day | ? | Interstitial pneumonitis | Recovered | Kim et al. (2018) | |
| 10 | 66 years | Male | ? mg/day | ? | Interstitial pneumonitis | Recovered | Derichs et al. (2018) | |
| 11 | 86 years | Male | 150 mg/day | 6 years | Eosinophilic pneumonitis | Recovered | Umeojiako & James (2019) | |
| Footnotes: a = Died of pneumothorax followed by spontaneous rupture of bulla induced by previous interstitial pneumonitis 14 months after discontinuation of bicalutamide and recovery from interstitial pneumonitis. Notes: Twelve additional cases of bicalutamide-associated interstitial pneumonitis, three of which resulted in death, were observed in an 87,000-patient cohort from MedWatch (U.S. FDA passive adverse-event reporting database) between 1998 and 2000 (0.01% incidence). The median age of the patients was 73.5 years (range 59 to 91 years), and median duration of bicalutamide exposure was 7.5 weeks (range 1 to 312 weeks). | ||||||||
Sensitivity to light
A few cases of photosensitivity (hypersensitivity to ultraviolet light-induced skin redness and/or lesions) associated with bicalutamide have been reported.[109][110][111] In one of the cases, bicalutamide was continued due to effectiveness in treating prostate cancer in the patient, and in combination with strict photoprotection (in the form of avoidance/prevention of ultraviolet light exposure). Eventually, the symptoms disappeared and did not recur.[109] Flutamide is also associated with photosensitivity, but much more frequently in comparison to bicalutamide.[109][111]
Male breast cancer
A case report of male breast cancer subsequent to bicalutamide-induced gynecomastia has been published.[112] According to the authors, "this is the second confirmed case of breast cancer in association with bicalutamide-induced gynaecomastia (correspondence AstraZeneca)."[112] It is notable, however, that gynecomastia does not seem to increase the risk of breast cancer in men.[112][113] Moreover, the lifetime incidence of breast cancer in men is approximately 0.1%,[114] the average age of diagnosis of prostate cancer and male breast cancer are similar (around 70 years),[30][115] and millions of men have been treated with bicalutamide for prostate cancer,[116] all of which are potentially in support of the notion of chance co-occurrences.[112] In accordance, the authors concluded that "causality cannot be established" and that it was "probable that the association is entirely coincidental and sporadic."[112]
Male birth defects
Because bicalutamide blocks the AR, like all antiandrogens, it can interfere with the androgen-mediated sexual differentiation of the genitalia (and brain) during prenatal development.[117][118][119][120] In pregnant rats given bicalutamide at a dosage of 10 mg/kg/day (resulting in circulating drug levels approximately equivalent to two-thirds of human therapeutic concentrations) and above, feminization of male offspring, such as reduced anogenital distance and hypospadias, as well as impotence, were observed.[77] No other teratogenic effects were observed in rats or rabbits receiving up to very high dosages of bicalutamide (that corresponded to up to approximately two times human therapeutic levels), and no teratogenic effects of any sort were observed in female rat offspring at any dosage.[77] As such, bicalutamide is a selective reproductive teratogen in males, and may have the potential to produce undervirilization/sexually ambiguous genitalia in male fetuses.[117][118]
References
- Mcleod DG (September 2002). "Emerging role of adjuvant hormonal therapy". Urology. 60 (3 Suppl 1): 13–20, discussion 21. doi:10.1016/S0090-4295(02)01562-5. PMID 12231039.
- https://pdf.hres.ca/dpd_pm/00009096.PDF
- Bennett CL, Raisch DW, Sartor O (October 2002). "Pneumonitis associated with nonsteroidal antiandrogens: presumptive evidence of a class effect". Annals of Internal Medicine. 137 (7): 625. doi:10.7326/0003-4819-137-7-200210010-00029. PMID 12353966.
An estimated 0.77% of the 6,480 nilutamide-treated patients, 0.04% of the 41,700 flutamide-treated patients, and 0.01% of the 86,800 bicalutamide-treated patients developed pneumonitis during the study period.
- Molina Mancero, Guillermo; Picón, Xavier; Di Tullio, Fernando; Ernst, Glenda; Dezanzo, Pablo; Salvado, Alejandro; Chertcoff, Julio F (2016). "Neumonía intersticial inducida por bloqueo androgénico máximo como tratamiento de cáncer de próstata avanzado" [Fatal interstitial lung disease associated with maximum androgen blockade. Report of one case]. Revista médica de Chile. 144 (10): 1356–1359. doi:10.4067/S0034-98872016001000017. ISSN 0034-9887.
- Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C (May 2016). "Drug-induced photosensitivity to bicalutamide - case report and review of the literature". Photodermatol Photoimmunol Photomed. 32 (3): 161–4. doi:10.1111/phpp.12230. PMID 26663090.
- Gretarsdottir, Helga M.; Bjornsdottir, Elin; Bjornsson, Einar S. (2018). "Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect". Case Reports in Gastroenterology. 12 (2): 266–270. doi:10.1159/000485175. ISSN 1662-0631.
- Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- Iversen P (December 2003). "Bicalutamide monotherapy for early stage prostate cancer: an update". J. Urol. 170 (6 Pt 2): S48–52, discussion S52–4. doi:10.1097/01.ju.0000096491.61731.38. PMID 14610410.
- Fourcade, R.-O.; McLeod, D. (2015). "Tolerability of Antiandrogens in the Treatment of Prostate Cancer". UroOncology. 4 (1): 5–13. doi:10.1080/1561095042000191655. ISSN 1561-0950.
- Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID (1998). "Casodex 10-200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group". Eur. Urol. 33 (1): 39–53. doi:10.1159/000019526. PMID 9471040.
- "Casodex Product Monograph" (PDF). Retrieved 24 September 2018.
- "NU-Bicalutamide Product Monograph" (PDF). Retrieved 24 September 2018.
- Blackledge GR (1996). "Clinical progress with a new antiandrogen, Casodex (bicalutamide)". Eur. Urol. 29 Suppl 2: 96–104. doi:10.1159/000473847. PMID 8717470.
- Michalopoulos NV, Keshtgar MR (2012). "Images in clinical medicine. Gynecomastia induced by prostate-cancer treatment". The New England Journal of Medicine. 367 (15): 1449. doi:10.1056/NEJMicm1209166. PMID 23050528.
Gynecomastia occurs in up to 80% of patients who receive nonsteroidal antiandrogens (eg, bicalutamide, flutamide, or nilutamide), usually within the first 6 to 9 months after the initiation of treatment.
- Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 150–152. ISBN 978-0-08-093292-7.
In contrast [to flutamide and nilutamide], no specific non-pharmacological complications have been linked to bicalutamide, while diarrhea and abnormal liver function occur less often than with flutamide.
- Fradet Y, Egerdie B, Andersen M, Tammela TL, Nachabe M, Armstrong J, Morris T, Navani S (2007). "Tamoxifen as prophylaxis for prevention of gynaecomastia and breast pain associated with bicalutamide 150 mg monotherapy in patients with prostate cancer: a randomised, placebo-controlled, dose-response study". European Urology. 52 (1): 106–14. doi:10.1016/j.eururo.2007.01.031. PMID 17270340.
- Wirth MP, Hakenberg OW, Froehner M (February 2007). "Antiandrogens in the treatment of prostate cancer". European Urology. 51 (2): 306–13, discussion 314. doi:10.1016/j.eururo.2006.08.043. PMID 17007995.
- Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer" (PDF). Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. Archived (PDF) from the original on 28 August 2016.
- Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554.
- Brown JS, Rubenfeld S (1974). "Irradiation in preventing gynecomastia induced by estrogens". Urology. 3 (1): 51–3. doi:10.1016/s0090-4295(74)80060-9. PMID 4812899.
Infrequently, the breast hypertrophy can become so marked that it attains proportions comparable to that in female breasts.
- Deepinder F, Braunstein GD (2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–95. doi:10.1517/14740338.2012.712109. PMID 22862307.
Treatment with estrogen has the highest incidence of gynecomastia, at 40 – 80%, anti-androgens, including flutamide, bicalutamide and nilutamide, are next, with a 40 – 70% incidence, followed by GnRH analogs (goserelin, leuprorelin) and combined androgen deprivation, both with incidences of 13% each.
- Nakabayashi M, Bartlett RA, Oh WK (2006). "Treatment of bicalutamide-induced gynecomastia with breast-reduction surgery in prostate cancer". Journal of Clinical Oncology. 24 (18): 2958–9. doi:10.1200/JCO.2005.03.8505. PMID 16782932.
- Saltzstein D, Sieber P, Morris T, Gallo J (2005). "Prevention and management of bicalutamide-induced gynecomastia and breast pain: randomized endocrinologic and clinical studies with tamoxifen and anastrozole". Prostate Cancer and Prostatic Diseases. 8 (1): 75–83. doi:10.1038/sj.pcan.4500782. PMID 15685254.
- Boccardo F, Rubagotti A, Battaglia M, Di Tonno P, Selvaggi FP, Conti G, Comeri G, Bertaccini A, Martorana G, Galassi P, Zattoni F, Macchiarella A, Siragusa A, Muscas G, Durand F, Potenzoni D, Manganelli A, Ferraris V, Montefiore F (February 2005). "Evaluation of tamoxifen and anastrozole in the prevention of gynecomastia and breast pain induced by bicalutamide monotherapy of prostate cancer". Journal of Clinical Oncology. 23 (4): 808–15. doi:10.1200/JCO.2005.12.013. PMID 15681525.
- Fagerlund A, Cormio L, Palangi L, Lewin R, Santanelli di Pompeo F, Elander A, Selvaggi G (2015). "Gynecomastia in Patients with Prostate Cancer: A Systematic Review". PLOS ONE. 10 (8): e0136094. Bibcode:2015PLoSO..1036094F. doi:10.1371/journal.pone.0136094. PMC 4550398. PMID 26308532.
- Di Lorenzo G, Autorino R, Perdonà S, De Placido S (2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- Fentiman IS (January 2018). "Managing Male Mammary Maladies". Eur J Breast Health. 14 (1): 5–9. doi:10.5152/ejbh.2017.3841. PMC 5758064. PMID 29322112.
- Fradet Y, Egerdie B, Andersen M, Tammela TL, Nachabe M, Armstrong J, Morris T, Navani S (July 2007). "Tamoxifen as prophylaxis for prevention of gynaecomastia and breast pain associated with bicalutamide 150 mg monotherapy in patients with prostate cancer: a randomised, placebo-controlled, dose-response study". Eur. Urol. 52 (1): 106–14. doi:10.1016/j.eururo.2007.01.031.
- Rahman HP, Hofland J, Foster PA (2016). "In touch with your feminine side: how oestrogen metabolism impacts prostate cancer" (PDF). Endocrine-Related Cancer. 23 (6): R249–66. doi:10.1530/ERC-16-0118. PMID 27194038.
Prostate cancer primarily affects the elderly with 99.9% of patients diagnosed over the age of 50 and the mean age at diagnosis being 73 (Parkin, et al. 1997).
- Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992.
- Migliari R, Muscas G, Usai E (August 1992). "Effect of Casodex on sleep-related erections in patients with advanced prostate cancer". J. Urol. 148 (2 Pt 1): 338–41. doi:10.1016/S0022-5347(17)36588-6. PMID 1378907.
- Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888.
- Paradisi R, Fabbri R, Porcu E, Battaglia C, Seracchioli R, Venturoli S (October 2011). "Retrospective, observational study on the effects and tolerability of flutamide in a large population of patients with acne and seborrhea over a 15-year period". Gynecol. Endocrinol. 27 (10): 823–9. doi:10.3109/09513590.2010.526664. PMID 21117864.
Among the slight and temporary adverse events [of flutamide], most frequently reported and not requesting treatment discontinuation were headache (7.8%), respiratory tract disorders (7.0%), nausea and/or vomiting (4.0%), diarrhea (4.0%), dry skin (9.5%), and reduction of libido (4.5%).
- Venturoli S, Paradisi R, Bagnoli A, Colombo FM, Ravaioli B, Vianello F, Mancini F, Gualerzi B, Porcu E, Seracchioli R (2001). [[...] changes in serum levels of the aminotransferases [11] or side effects (stomach pain, headache, dry skin, nausea, increased appetite, decrease of libido) are only occasionally seen [with flutamide] [10, 11]. "Low-dose flutamide (125 mg/day) as maintenance therapy in the treatment of hirsutism"] Check
|url=value (help). Horm. Res. 56 (1–2): 25–31. doi:10.1159/000048086. PMID 11815724. - Reed BG, Bou Nemer L, Carr BR (2016). "Has testosterone passed the test in premenopausal women with low libido? A systematic review". Int J Women's Health. 8: 599–607. doi:10.2147/IJWH.S116212. PMC 5066846. PMID 27785108.
- Cappelletti M, Wallen K (February 2016). "Increasing women's sexual desire: The comparative effectiveness of estrogens and androgens". Horm Behav. 78: 178–93. doi:10.1016/j.yhbeh.2015.11.003. PMC 4720522. PMID 26589379.
- Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- Morgante E, Gradini R, Realacci M, Sale P, D'Eramo G, Perrone GA, Cardillo MR, Petrangeli E, Russo M, Di Silverio F (March 2001). "Effects of long-term treatment with the anti-androgen bicalutamide on human testis: an ultrastructural and morphometric study". Histopathology. 38 (3): 195–201. doi:10.1046/j.1365-2559.2001.01077.x. PMID 11260298.
- Higano CS (2012). "Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer". Journal of Clinical Oncology. 30 (30): 3720–5. doi:10.1200/JCO.2012.41.8509. PMID 23008326.
- Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR (May 2015). "Adverse effects of androgen deprivation therapy and strategies to mitigate them". European Urology. 67 (5): 825–36. doi:10.1016/j.eururo.2014.07.010. PMID 25097095.
- Mazzola CR, Mulhall JP (March 2012). "Impact of androgen deprivation therapy on sexual function". Asian Journal of Andrology. 14 (2): 198–203. doi:10.1038/aja.2011.106. PMC 3735098. PMID 22231298.
- Mulcahy JJ (1 January 2001). Male Sexual Function. Springer Science & Business Media. pp. 3–. ISBN 978-1-59259-098-8. Archived from the original on 20 May 2016.
- Bjerklund Johansen TE, Majak M, Nesland JM (March 1994). "Testicular histology after treatment with the new antiandrogen Casodex for carcinoma of the prostate. A preliminary report". Scand. J. Urol. Nephrol. 28 (1): 67–70. doi:10.3109/00365599409180473. PMID 8009196.
- Scialli AR, Clegg ED (9 June 1992). Reversibility in Testicular Toxicity Assessment. CRC Press. pp. 107–. ISBN 978-0-8493-5980-4.
- Kolvenbag GJ, Blackledge GR (January 1996). "Worldwide activity and safety of bicalutamide: a summary review". Urology. 47 (1A Suppl): 70–9, discussion 80–4. doi:10.1016/s0090-4295(96)80012-4. PMID 8560681.
Bicalutamide is a new antiandrogen that offers the convenience of once-daily administration, demonstrated activity in prostate cancer, and an excellent safety profile. Because it is effective and offers better tolerability than flutamide, bicalutamide represents a valid first choice for antiandrogen therapy in combination with castration for the treatment of patients with advanced prostate cancer.
- Jones HB, Betton GR, Bowdler AL, McFarquhar RL, Middleton BJ, Lunglmayr G (1994). "Pathological and morphometric assessment of testicular parameters in patients with metastatic prostate cancer following treatment with either the antiandrogen Casodex (ZM176,334) or bilateral orchidectomy". Urol. Res. 22 (3): 191–5. doi:10.1007/BF00571849. PMID 7992465.
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Van Poppel H, Tammela TL, Chamberlain M, Carroll K, Melezinek I (2000). "Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup". The Journal of Urology. 164 (5): 1579–82. doi:10.1016/s0022-5347(05)67032-2. PMID 11025708.
- Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Carroll K (November 2004). "Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6". The Journal of Urology. 172 (5 Pt 1): 1871–6. doi:10.1097/01.ju.0000139719.99825.54. PMID 15540741.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 34, 55. ISBN 978-0-9828280-1-4.
- Mangus BC, Miller MG (11 January 2005). Pharmacology Application in Athletic Training. F.A. Davis. pp. 151–. ISBN 978-0-8036-2027-8.
- Oh WK (2001). "Anemia Related to Hormonal Ablation Therapy for Prostate Cancer". The Prostate Journal. 3 (1): 14–17. doi:10.1046/j.1525-1411.2001.003001014.x.
- Droz J, Audisio RA (2 October 2012). Management of Urological Cancers in Older People. Springer Science & Business Media. pp. 84–. ISBN 978-0-85729-986-4. Archived from the original on 11 May 2016.
- Mason M (August 2006). "What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care?". Journal of Cancer Research and Clinical Oncology. 132 Suppl 1: S27–35. doi:10.1007/s00432-006-0134-4. PMID 16896883.
- Cher ML, Honn KV, Raz A (11 April 2006). Prostate Cancer: New Horizons in Research and Treatment. Springer Science & Business Media. pp. 382–. ISBN 978-0-306-48143-7. Archived from the original on 5 May 2016.
- Feldman D, Marcus R, Nelson D, Rosen CJ (8 November 2007). Osteoporosis. Academic Press. pp. 1354–. ISBN 978-0-08-055347-4. Archived from the original on 11 June 2016.
- Vanderschueren D, Gaytant J, Boonen S, Venken K (June 2008). "Androgens and bone". Current Opinion in Endocrinology, Diabetes and Obesity. 15 (3): 250–4. doi:10.1097/MED.0b013e3282fe6ca9. PMID 18438173.
- Bowsher W, Carter A (15 April 2008). Challenges in Prostate Cancer. John Wiley & Sons. pp. 146–. ISBN 978-1-4051-7177-9.
- Shahani R, Fleshner NE, Zlotta AR (2007). "Pharmacotherapy for prostate cancer: the role of hormonal treatment". Discovery Medicine. 7 (39): 118–24. PMID 18093474.
- Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schröder FH, Sternberg CN, Studer UE (2012). "Contemporary role of androgen deprivation therapy for prostate cancer". European Urology. 61 (1): 11–25. doi:10.1016/j.eururo.2011.08.026. PMC 3483081. PMID 21871711.
- Sternberg CN (2006). "Adjuvant bicalutamide for early prostate cancer: an update". Nature Clinical Practice Urology. 3 (8): 408–9. doi:10.1038/ncpuro0518. PMID 16902511.
- Stanworth RD, Jones TH (2008). "Testosterone for the aging male; current evidence and recommended practice". Clinical Interventions in Aging. 3 (1): 25–44. doi:10.2147/CIA.S190. PMC 2544367. PMID 18488876.
- Iversen P, Johansson JE, Lodding P, Kylmälä T, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Armstrong J (2006). "Bicalutamide 150 mg in addition to standard care for patients with early non-metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group-6 Study after a median follow-up period of 7.1 years". Scandinavian Journal of Urology and Nephrology. 40 (6): 441–52. doi:10.1080/00365590601017329. PMID 17130095.
- Chodak G, Gomella L, Phung de H (September 2007). "Combined androgen blockade in advanced prostate cancer: looking back to move forward". Clin Genitourin Cancer. 5 (6): 371–8. doi:10.3816/CGC.2007.n.019. PMID 17956709.
- Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T (September 2006). "Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration". BJU Int. 98 (3): 563–72. doi:10.1111/j.1464-410X.2006.06275.x. PMID 16771791.
- Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, Beckman JA, Choueiri TK (2011). "Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials". JAMA: The Journal of the American Medical Association. 306 (21): 2359–66. doi:10.1001/jama.2011.1745. PMID 22147380.
- Guirguis K (2016). "Bicalutamide causes heart failure in an elderly patient with prostate cancer". Expert Opin Drug Saf. 15 (3): 297–302. doi:10.1517/14740338.2015.1131819. PMID 26745594.
- Peng CC, Chen CY, Chen CR, Chen CJ, Shen KH, Chen KC, Peng RY (March 2019). "Renal Damaging Effect Elicited by Bicalutamide Therapy Uncovered Multiple Action Mechanisms As Evidenced by the Cell Model". Sci Rep. 9 (1): 3392. Bibcode:2019NatSR...9.3392P. doi:10.1038/s41598-019-39533-3. PMC 6399217. PMID 30833616.
- Hussain S, Haidar A, Bloom RE, Zayouna N, Piper MH, Jafri SM (2014). "Bicalutamide-induced hepatotoxicity: A rare adverse effect". Am J Case Rep. 15: 266–70. doi:10.12659/AJCR.890679. PMC 4068966. PMID 24967002.
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, et al. (August 2002). "Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program". The Journal of Urology. 168 (2): 429–35. doi:10.1016/S0022-5347(05)64652-6. PMID 12131282.
- Craig JV, Furr B (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 356–. ISBN 978-1-59259-152-7.
A case of near-fatal fulminant hepatic failure in a patient on bicalutamide therapy (50 mg) has recently been published (101), but it is uncertain whether this can be attributed to bicalutamide, as the symptoms developed after only two doses in a patient previously exposed to both cyproterone acetate and flutamide (101).
- Kaplowitz N (16 October 2002). Drug-Induced Liver Disease. CRC Press. pp. 618–. ISBN 978-0-203-90912-6.
- Kim JH, Yoo BW, Yang WJ (May 2014). "Hepatic failure induced by cyproterone acetate: A case report and literature review". Canadian Urological Association Journal. 8 (5–6): E458–61. doi:10.5489/cuaj.1753. PMC 4081269. PMID 25024808.
- Savidou I, Deutsch M, Soultati AS, Koudouras D, Kafiri G, Dourakis SP (December 2006). "Hepatotoxicity induced by cyproterone acetate: a report of three cases". World Journal of Gastroenterology. 12 (46): 7551–5. doi:10.3748/wjg.v12.i46.7551. PMC 4087608. PMID 17167851.
- Keating GM (March 2015). "Enzalutamide: a review of its use in chemotherapy-naïve metastatic castration-resistant prostate cancer". Drugs & Aging. 32 (3): 243–9. doi:10.1007/s40266-015-0248-y. PMID 25711765.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B (July 2014). "Enzalutamide in metastatic prostate cancer before chemotherapy". The New England Journal of Medicine. 371 (5): 424–33. doi:10.1056/NEJMoa1405095. PMC 4418931. PMID 24881730.
- "Casodex® (bicalutamide) Tablets" (PDF). FDA. Archived (PDF) from the original on 27 February 2017.
- Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- Lehne RA (2013). Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 1297–. ISBN 978-1-4377-3582-6.
- Tripathi KD (30 September 2013). Essentials of Medical Pharmacology. JP Medical Ltd. pp. 302–. ISBN 978-93-5025-937-5.
- Gretarsdottir, Helga M.; Bjornsdottir, Elin; Bjornsson, Einar S. (2018). "Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect". Case Reports in Gastroenterology. 12 (2): 266–270. doi:10.1159/000485175. ISSN 1662-0631.
- Yun GY, Kim SH, Kim SW, Joo JS, Kim JS, Lee ES, Lee BS, Kang SH, Moon HS, Sung JK, Lee HY, Kim KH (April 2016). "Atypical onset of bicalutamide-induced liver injury". World J. Gastroenterol. 22 (15): 4062–5. doi:10.3748/wjg.v22.i15.4062. PMC 4823258. PMID 27099451.
- Danseuse P, Snyder RR, Monks TJ, Jollow DJ, Sipes IG, Greim H, Gibson GG, Delaforge M (6 December 2012). Biological Reactive Intermediates Vi: Chemical and Biological Mechanisms in Susceptibility to and Prevention of Environmental Diseases. Springer Science & Business Media. pp. 37–. ISBN 978-1-4615-0667-6. Archived from the original on 28 April 2016.
- Schellhammer PF (November 1997). "Fulminant hepatic failure associated with bicalutamide". Urology. 50 (5): 827. doi:10.1016/S0090-4295(97)80116-1. PMID 9372905.
- O'Bryant CL, Flaig TW, Utz KJ (August 2008). "Bicalutamide-associated fulminant hepatotoxicity". Pharmacotherapy. 28 (8): 1071–5. doi:10.1592/phco.28.8.1071. PMID 18657023.
- Jordan VC, Furr BJ (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 350–. ISBN 978-1-59259-152-7. Archived from the original on 29 May 2016.
- Ramon J, Denis L (5 June 2007). Prostate Cancer. Springer Science & Business Media. pp. 256–. ISBN 978-3-540-40901-4. Archived from the original on 27 April 2016.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 169–. ISBN 978-1-60913-345-0.
- Vinod Rustgi (16 November 2016). Drug Hepatotoxicity, An Issue of Clinics in Liver Disease, E-Book. Elsevier Health Sciences. pp. 89–. ISBN 978-0-323-49662-9.
- Giorgetti R, di Muzio M, Giorgetti A, Girolami D, Borgia L, Tagliabracci A (2017). "Flutamide-induced hepatotoxicity: ethical and scientific issues". European Reviews for Medical and Pharmacological Sciences. 21 (1 Suppl): 69–77. PMID 28379593.
- Bunce CM, Campbell MJ (11 March 2010). Nuclear Receptors: Current Concepts and Future Challenges. Springer Science & Business Media. pp. 160, 167. ISBN 978-90-481-3303-1. Archived from the original on 10 June 2016.
- Coe KJ, Jia Y, Ho HK, Rademacher P, Bammler TK, Beyer RP, Farin FM, Woodke L, Plymate SR, Fausto N, Nelson SD (September 2007). "Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening". Chemical Research in Toxicology. 20 (9): 1277–90. doi:10.1021/tx7001349. PMC 2802183. PMID 17702527.
- Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y (June 2007). "Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants". Toxicological Sciences. 97 (2): 539–47. doi:10.1093/toxsci/kfm052. PMID 17361016.
Apoptosis induced by the androgen antagonist bicalutamide is receptor mediated (Lin et al., 2006), and hence a dominant effect at low concentrations, and hepatoxicity is a rare event (Dawson et al., 1997), in accord with its relative lack of toxicity to galactose-grown cells.
- Kashimshetty R, Desai VG, Kale VM, Lee T, Moland CL, Branham WS, New LS, Chan EC, Younis H, Boelsterli UA (July 2009). "Underlying mitochondrial dysfunction triggers flutamide-induced oxidative liver injury in a mouse model of idiosyncratic drug toxicity". Toxicology and Applied Pharmacology. 238 (2): 150–9. doi:10.1016/j.taap.2009.05.007. PMID 19442681.
- Ball AL, Kamalian L, Alfirevic A, Lyon JJ, Chadwick AE (July 2016). "Identification of the Additional Mitochondrial Liabilities of 2-Hydroxyflutamide When Compared With its Parent Compound, Flutamide in HepG2 Cells". Toxicological Sciences. 153 (2): 341–351. doi:10.1093/toxsci/kfw126. PMC 5036617. PMID 27413113.
- Boelsterli UA, Ho HK, Zhou S, Leow KY (October 2006). "Bioactivation and hepatotoxicity of nitroaromatic drugs". Current Drug Metabolism. 7 (7): 715–27. doi:10.2174/138920006778520606. PMID 17073576.
- "Drug Record: Bicalutamide - LiverTox" (HTML). National Library of Medicine. National Institutes of Health. Retrieved 13 November 2018.
- Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497–. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- Masago T, Watanabe T, Nemoto R, Motoda K (December 2011). "Interstitial pneumonitis induced by bicalutamide given for prostate cancer". International Journal of Clinical Oncology. 16 (6): 763–5. doi:10.1007/s10147-011-0239-x. PMID 21537882.
- Aronson JK (4 March 2014). Side Effects of Drugs Annual: A worldwide yearly survey of new data in adverse drug reactions. Newnes. pp. 740–. ISBN 978-0-444-62636-3. Archived from the original on 6 May 2016.
- Umeojiako WI, James M (2019). "Bicalutamide-induced Eosinophilic Pneumonitis-A Serendipitous Diagnosis". Journal of Case Reports in Medicine. 8 (1): 6. doi:10.25149/case-reports.v8i1.164. ISSN 2090-5351.
- Ricci F, Buzzatti G, Rubagotti A, Boccardo F (November 2014). "Safety of antiandrogen therapy for treating prostate cancer". Expert Opinion on Drug Safety. 13 (11): 1483–99. doi:10.1517/14740338.2014.966686. PMID 25270521.
- Gulley JL (2011). Prostate Cancer. Demos Medical Publishing. pp. 81–. ISBN 978-1-935281-91-7. Archived from the original on 25 April 2016.
- Camus P, Rosenow III EC (29 October 2010). Drug-induced and Iatrogenic Respiratory Disease. CRC Press. pp. 235–. ISBN 978-1-4441-2869-7.
- Rodriguez EM, Staffa JA, Graham DJ (2001). "The role of databases in drug postmarketing surveillance". Pharmacoepidemiology and Drug Safety. 10 (5): 407–10. doi:10.1002/pds.615. PMID 11802586.
- Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497, 521. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- Wong PW, Macris N, DiFabrizio L, Seriff NS (February 1998). "Eosinophilic lung disease induced by bicalutamide: a case report and review of the medical literature". Chest. 113 (2): 548–50. doi:10.1378/chest.113.2.548. PMID 9498983.
- Daba MH, El-Tahir KE, Al-Arifi MN, Gubara OA (June 2004). "Drug-induced pulmonary fibrosis". Saudi Medical Journal. 25 (6): 700–6. PMID 15195196.
- Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C (May 2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Photodermatology, Photoimmunology & Photomedicine. 32 (3): 161–4. doi:10.1111/phpp.12230. PMID 26663090.
- Lee K, et al. (2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Reactions Weekly. 1612 (1): 37. doi:10.1007/s40278-016-19790-1.
- Sasada K, Sakabe J, Tamura A, Kasuya A, Shimauchi T, Ito T, Hirakawa S, Tokura Y (2012). "Photosensitive drug eruption induced by bicalutamide within the UVB action spectrum". European Journal of Dermatology. 22 (3): 402–3. doi:10.1684/ejd.2012.1719. PMID 22503957.
- Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 155–. ISBN 978-0-08-093292-7.
- Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B (March 2014). "Gynecomastia: Clinical evaluation and management". Indian Journal of Endocrinology and Metabolism. 18 (2): 150–8. doi:10.4103/2230-8210.129104. PMC 3987263. PMID 24741509.
- Nussbaum RL, McInnes RR, Willard HF (21 May 2015). Thompson & Thompson Genetics in Medicine. Elsevier Health Sciences. pp. 319–. ISBN 978-1-4377-0696-3.
- Christopher Li (11 November 2009). Breast Cancer Epidemiology. Springer Science & Business Media. pp. 261–. ISBN 978-1-4419-0685-4.
- Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016
- Iswaran TJ, Imai M, Betton GR, Siddall RA (May 1997). "An overview of animal toxicology studies with bicalutamide (ICI 176,334)". The Journal of Toxicological Sciences. 22 (2): 75–88. doi:10.2131/jts.22.2_75. PMID 9198005.
- Smith RE (4 April 2013). Medicinal Chemistry – Fusion of Traditional and Western Medicine. Bentham Science Publishers. pp. 306–. ISBN 978-1-60805-149-6. Archived from the original on 29 May 2016.
- Sex Differences in the Human Brain, their underpinnings and implications. Elsevier. 3 December 2010. pp. 44–45. ISBN 978-0-444-53631-0. Archived from the original on 26 May 2016.
- Paoletti R (6 December 2012). Chemistry and Brain Development: Proceedings of the Advanced Study Institute on "Chemistry of Brain Development," held in Milan, Italy, September 9–19, 1970. Springer Science & Business Media. pp. 218–. ISBN 978-1-4684-7236-3.