Neuroplasticity
Neuroplasticity, also known as brain plasticity, neuroelasticity, or neural plasticity, is the ability of the brain to change continuously throughout an individual's life, e.g., brain activity associated with a given function can be transferred to a different location, the proportion of grey matter can change, and synapses may strengthen or weaken over time. The aim of neuroplasticity is to optimize the neural networks during phylogenesis, ontogeny, and physiological learning, as well as after a brain injury.[1] Research in the latter half of the 20th century showed that many aspects of the brain can be altered (or are "plastic") even through adulthood.[2][3][4][5] However, the developing brain exhibits a higher degree of plasticity than the adult brain.[6][7]:30
Neuroplasticity can be observed at multiple scales, from microscopic changes in individual neurons to larger-scale changes such as cortical remapping in response to injury.[8] Behavior, environmental stimuli, thought, and emotions may also cause neuroplastic change through activity-dependent plasticity, which has significant implications for healthy development, learning, memory, and recovery from brain damage.[8][9][10] At the single cell level, synaptic plasticity refers to changes in the connections between neurons, whereas non-synaptic plasticity refers to changes in their intrinsic excitability.
History
Origin
The term "plasticity" was first applied to behavior in 1890 by William James in The Principles of Psychology.[11] The first person to use the term neural plasticity appears to have been the Polish neuroscientist Jerzy Konorski.[3][12]
In 1793, Italian anatomist Michele Vicenzo Malacarne described experiments in which he paired animals, trained one of the pair extensively for years, and then dissected both. He discovered that the cerebellums of the trained animals were substantially larger. But these findings were eventually forgotten.[13] The idea that the brain and its function are not fixed throughout adulthood was proposed in 1890 by William James in The Principles of Psychology, though the idea was largely neglected.[11] Until around the 1970s, neuroscientists believed that the brain's structure and function was essentially fixed throughout adulthood.[14]
While the brain was commonly understood as a nonrenewable organ in the early 1900s, Santiago Ramón y Cajal, father of neuroscience, used the term neuronal plasticity to describe nonpathological changes in the structure of adult brains. Based on his renowned Neuron Doctrine, Cajal first described the neuron as the fundamental unit of the nervous system that later served as an essential foundation to develop the concept of neural plasticity. [15] He used the term plasticity in reference to his work on findings of degeneration and regeneration in the central nervous system after a person had reached adulthood, specifically. Many neuroscientists used the term plasticity only to explain the regenerative capacity of the peripheral nervous system, which Cajal's conceptual transfer of the term gave rise to a controversial discussion.[16]
The term has since been broadly applied:
Given the central importance of neuroplasticity, an outsider would be forgiven for assuming that it was well defined and that a basic and universal framework served to direct current and future hypotheses and experimentation. Sadly, however, this is not the case. While many neuroscientists use the word neuroplasticity as an umbrella term it means different things to different researchers in different subfields ... In brief, a mutually agreed upon framework does not appear to exist.[17]
Research and discovery
In 1923, Karl Lashley conducted experiments on rhesus monkeys that demonstrated changes in neuronal pathways, which he concluded were evidence of plasticity. Despite this, and other research that suggested plasticity took place, neuroscientists did not widely accept the idea of neuroplasticity.
In 1945, Justo Gonzalo concluded from his research of brain dynamics, that, contrary to the activity of the projection areas, the "central" cortical mass (more or less equidistant from the visual, tactile and auditive projection areas), would be a "maneuvering mass", rather unspecific or multisensory, with capacity to increase neural excitability and re-organize the activity by means of plasticity properties.[18] He gives as a first example of adaptation, to see upright with reversing glasses in the Stratton experiment,[19] and specially, several first-hand brain injuries cases in which he observed dynamic and adaptive properties in their disorders, in particular in the inverted perception disorder [e.g., see pp 260–62 Vol. I (1945), p 696 Vol. II (1950)].[18] He stated that a sensory signal in a projection area would be only an inverted and constricted outline that would be magnified due to the increase in recruited cerebral mass, and re-inverted due to some effect of brain plasticity, in more central areas, following a spiral growth.[20]
Marian Diamond of the University of California, Berkeley, produced the first scientific evidence of anatomical brain plasticity, publishing her research in 1964.[21][2]
Other significant evidence was produced in the 1960s and after, notably from scientists including Paul Bach-y-Rita, Michael Merzenich along with Jon Kaas, as well as several others.[14][22]
In the 1960s, Paul Bach-y-Rita invented a device that was tested on a small number of people, and involved a person sitting in a chair, in which were embedded nubs that were made to vibrate in ways that translated images received in a camera, allowing a form of vision via sensory substitution.[23][24]
Studies in people recovering from stroke also provided support for neuroplasticity, as regions of the brain remained healthy could sometimes take over, at least in part, functions that had been destroyed; Shepherd Ivory Franz did work in this area.[25][26]
Eleanor Maguire documented changes in hippocampal structure associated with acquiring the knowledge of London's layout in local taxi drivers.[27][28][29] A redistribution of grey matter was indicated in London Taxi Drivers compared to controls. This work on hippocampal plasticity not only interested scientists, but also engaged the public and media worldwide.
Michael Merzenich is a neuroscientist who has been one of the pioneers of neuroplasticity for over three decades. He has made some of "the most ambitious claims for the field – that brain exercises may be as useful as drugs to treat diseases as severe as schizophrenia – that plasticity exists from cradle to the grave, and that radical improvements in cognitive functioning – how we learn, think, perceive, and remember are possible even in the elderly."[23] Merzenich's work was affected by a crucial discovery made by David Hubel and Torsten Wiesel in their work with kittens. The experiment involved sewing one eye shut and recording the cortical brain maps. Hubel and Wiesel saw that the portion of the kitten's brain associated with the shut eye was not idle, as expected. Instead, it processed visual information from the open eye. It was "…as though the brain didn't want to waste any 'cortical real estate' and had found a way to rewire itself."[23]
This implied neuroplasticity during the critical period. However, Merzenich argued that neuroplasticity could occur beyond the critical period. His first encounter with adult plasticity came when he was engaged in a postdoctoral study with Clinton Woosley. The experiment was based on observation of what occurred in the brain when one peripheral nerve was cut and subsequently regenerated. The two scientists micromapped the hand maps of monkey brains before and after cutting a peripheral nerve and sewing the ends together. Afterwards, the hand map in the brain that they expected to be jumbled was nearly normal. This was a substantial breakthrough. Merzenich asserted that, "If the brain map could normalize its structure in response to abnormal input, the prevailing view that we are born with a hardwired system had to be wrong. The brain had to be plastic."[23] Merzenich received the 2016 Kavli Prize in Neuroscience "for the discovery of mechanisms that allow experience and neural activity to remodel brain function."[30]
Neurobiology
JT Wall and J Xu have traced the mechanisms underlying neuroplasticity. Re-organization is not cortically emergent, but occurs at every level in the processing hierarchy; this produces the map changes observed in the cerebral cortex.[31]
Types
As Shaw and McEachern described above, there is no all-inclusive theory that overarches different frameworks and systems in the study of neuroplasticity. However, researchers often describe neuroplasticity as “the ability to make adaptive changes related to the structure and function of the nervous system."[32] Correspondingly, two types of neuroplasticity are often discussed: structural neuroplasticity and functional neuroplasticity.
Structural neuroplasticity
Structural plasticity is often understood as the brain's ability to change its neuronal connections. New neurons are constantly produced and integrated into the central nervous system throughout the life span based on this type of neuroplasticity. Researchers nowadays use multiple cross-sectional imaging methods (i.e. magnetic resonance imaging (MRI), computerized tomography (CT)) to study the structural alterations of the human brains.[33] This type of neuroplasticity often studies the effect of various internal or external stimuli on the brain’s anatomical reorganization. The changes of grey matter proportion or the synaptic strength in the brain are considered as examples of structural neuroplasticity. Structural neuroplasticity is currently investigated more within the field of neuroscience in current academia.[34]
Functional neuroplasticity
Functional plasticity (also known as synaptic plasticity) refers to brain's ability to alter and adapt the communication between neurons. The changes often occur due to but not limited to the malfunction or damage of neurons; the functions from one part of the brain transfer to another part of the brain based on the demand to produce recovery of behavioral or physiological processes.[35] Long-term potentiation (LTP) and long-term depression (LTD) are considered as examples of synaptic plasticity that are associated with memory.[36] Studies have shown that different life experience such as juggling, video-game playing, or musical training is associated with functional plasticity.
Applications and example
The adult brain is not entirely "hard-wired" with fixed neuronal circuits. There are many instances of cortical and subcortical rewiring of neuronal circuits in response to training as well as in response to injury. There is solid evidence that neurogenesis (birth of brain cells) occurs in the adult, mammalian brain—and such changes can persist well into old age.[4] The evidence for neurogenesis is mainly restricted to the hippocampus and olfactory bulb, but current research has revealed that other parts of the brain, including the cerebellum, may be involved as well.[37] However, the degree of rewiring induced by the integration of new neurons in the established circuits is not known, and such rewiring may well be functionally redundant.[38]
There is now ample evidence for the active, experience-dependent re-organization of the synaptic networks of the brain involving multiple inter-related structures including the cerebral cortex. The specific details of how this process occurs at the molecular and ultrastructural levels are topics of active neuroscience research. The way experience can influence the synaptic organization of the brain is also the basis for a number of theories of brain function including the general theory of mind and Neural Darwinism. The concept of neuroplasticity is also central to theories of memory and learning that are associated with experience-driven alteration of synaptic structure and function in studies of classical conditioning in invertebrate animal models such as Aplysia.
Treatment of brain damage
A surprising consequence of neuroplasticity is that the brain activity associated with a given function can be transferred to a different location; this can result from normal experience and also occurs in the process of recovery from brain injury. Neuroplasticity is the fundamental issue that supports the scientific basis for treatment of acquired brain injury with goal-directed experiential therapeutic programs in the context of rehabilitation approaches to the functional consequences of the injury.
Neuroplasticity is gaining popularity as a theory that, at least in part, explains improvements in functional outcomes with physical therapy post-stroke. Rehabilitation techniques that are supported by evidence which suggest cortical reorganization as the mechanism of change include constraint-induced movement therapy, functional electrical stimulation, treadmill training with body-weight support, and virtual reality therapy. Robot assisted therapy is an emerging technique, which is also hypothesized to work by way of neuroplasticity, though there is currently insufficient evidence to determine the exact mechanisms of change when using this method.[39]
One group has developed a treatment that includes increased levels of progesterone injections in brain-injured patients. "Administration of progesterone after traumatic brain injury[40] (TBI) and stroke reduces edema, inflammation, and neuronal cell death, and enhances spatial reference memory and sensory motor recovery."[41] In a clinical trial, a group of severely injured patients had a 60% reduction in mortality after three days of progesterone injections.[42] However, a study published in the New England Journal of Medicine in 2014 detailing the results of a multi-center NIH-funded phase III clinical trial of 882 patients found that treatment of acute traumatic brain injury with the hormone progesterone provides no significant benefit to patients when compared with placebo.[43]
Vision
For decades, researchers assumed that humans had to acquire binocular vision, in particular stereopsis, in early childhood or they would never gain it. In recent years, however, successful improvements in persons with amblyopia, convergence insufficiency or other stereo vision anomalies have become prime examples of neuroplasticity; binocular vision improvements and stereopsis recovery are now active areas of scientific and clinical research.[44][45][46]
Brain training
Several companies have offered so-called cognitive training software programs for various purposes that claim to work via neuroplasticity; one example is Fast ForWord which is marketed to help children with learning disabilities.[47] A systematic meta-analytic review found that "There is no evidence from the analysis carried out that Fast ForWord is effective as a treatment for children's oral language or reading difficulties".[47] An official response to this review by the company points out that this review only examined 2 out of 11 Fast ForWord products, and further, that these 2 are outdated versions of their current counterparts.[48] More pointedly, it states, "The publication is misleading in several ways. Most of the studies that were selected had very poor implementations, and therefore are not representative of performance improvements following proper usage.... Strong et al. applied extremely restrictive study selection criteria to a corpus of more than two hundred studies on eleven Fast ForWord products – only six studies were included in their report and only five were included in their meta-analysis."[48]
Sensory prostheses
Neuroplasticity is involved in the development of sensory function. The brain is born immature and it adapts to sensory inputs after birth. In the auditory system, congenital hearing impairment, a rather frequent inborn condition affecting 1 of 1000 newborns, has been shown to affect auditory development, and implantation of a sensory prostheses activating the auditory system has prevented the deficits and induced functional maturation of the auditory system.[49] Due to a sensitive period for plasticity, there is also a sensitive period for such intervention within the first 2–4 years of life. Consequently, in prelingually deaf children, early cochlear implantation, as a rule, allows the children to learn the mother language and acquire acoustic communication.[50]
Phantom limbs
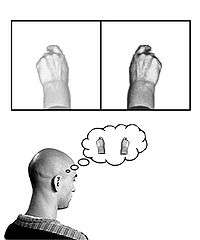
In the phenomenon of phantom limb sensation, a person continues to feel pain or sensation within a part of their body that has been amputated. This is strangely common, occurring in 60–80% of amputees.[51] An explanation for this is based on the concept of neuroplasticity, as the cortical maps of the removed limbs are believed to have become engaged with the area around them in the postcentral gyrus. This results in activity within the surrounding area of the cortex being misinterpreted by the area of the cortex formerly responsible for the amputated limb.
The relationship between phantom limb sensation and neuroplasticity is a complex one. In the early 1990s V.S. Ramachandran theorized that phantom limbs were the result of cortical remapping. However, in 1995 Herta Flor and her colleagues demonstrated that cortical remapping occurs only in patients who have phantom pain.[52] Her research showed that phantom limb pain (rather than referred sensations) was the perceptual correlate of cortical reorganization.[53] This phenomenon is sometimes referred to as maladaptive plasticity.
In 2009 Lorimer Moseley and Peter Brugger carried out a remarkable experiment in which they encouraged arm amputee subjects to use visual imagery to contort their phantom limbs into impossible configurations. Four of the seven subjects succeeded in performing impossible movements of the phantom limb. This experiment suggests that the subjects had modified the neural representation of their phantom limbs and generated the motor commands needed to execute impossible movements in the absence of feedback from the body.[54] The authors stated that: "In fact, this finding extends our understanding of the brain's plasticity because it is evidence that profound changes in the mental representation of the body can be induced purely by internal brain mechanisms—the brain truly does change itself."
Chronic pain
Individuals who suffer from chronic pain experience prolonged pain at sites that may have been previously injured, yet are otherwise currently healthy. This phenomenon is related to neuroplasticity due to a maladaptive reorganization of the nervous system, both peripherally and centrally. During the period of tissue damage, noxious stimuli and inflammation cause an elevation of nociceptive input from the periphery to the central nervous system. Prolonged nociception from the periphery then elicits a neuroplastic response at the cortical level to change its somatotopic organization for the painful site, inducing central sensitization.[55] For instance, individuals experiencing complex regional pain syndrome demonstrate a diminished cortical somatotopic representation of the hand contralaterally as well as a decreased spacing between the hand and the mouth.[56] Additionally, chronic pain has been reported to significantly reduce the volume of grey matter in the brain globally, and more specifically at the prefrontal cortex and right thalamus.[57] However, following treatment, these abnormalities in cortical reorganization and grey matter volume are resolved, as well as their symptoms. Similar results have been reported for phantom limb pain,[58] chronic low back pain[59] and carpal tunnel syndrome.[60]
Meditation
A number of studies have linked meditation practice to differences in cortical thickness or density of gray matter.[5][61][62][63] One of the most well-known studies to demonstrate this was led by Sara Lazar, from Harvard University, in 2000.[64] Richard Davidson, a neuroscientist at the University of Wisconsin, has led experiments in cooperation with the Dalai Lama on effects of meditation on the brain. His results suggest that long-term or short-term practice of meditation results in different levels of activity in brain regions associated with such qualities as attention, anxiety, depression, fear, anger, and the ability of the body to heal itself. These functional changes may be caused by changes in the physical structure of the brain.[65][66][67][68]
Fitness and exercise
Aerobic exercise promotes adult neurogenesis by increasing the production of neurotrophic factors (compounds that promote growth or survival of neurons), such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF).[69][70][71] Exercise-induced neurogenesis in the hippocampus is associated with measurable improvements in spatial memory.[72][73][74][75] Consistent aerobic exercise over a period of several months induces marked clinically significant improvements in executive function (i.e., the "cognitive control" of behavior) and increased gray matter volume in multiple brain regions, particularly those that give rise to cognitive control.[71][72][76][77] The brain structures that show the greatest improvements in gray matter volume in response to aerobic exercise are the prefrontal cortex and hippocampus;[71][72][73] moderate improvements are seen in the anterior cingulate cortex, parietal cortex, cerebellum, caudate nucleus, and nucleus accumbens.[71][72][73] Higher physical fitness scores (measured by VO2 max) are associated with better executive function, faster processing speed, and greater volume of the hippocampus, caudate nucleus, and nucleus accumbens.[72]
Human echolocation
Human echolocation is a learned ability for humans to sense their environment from echoes. This ability is used by some blind people to navigate their environment and sense their surroundings in detail. Studies in 2010[78] and 2011[79] using functional magnetic resonance imaging techniques have shown that parts of the brain associated with visual processing are adapted for the new skill of echolocation. Studies with blind patients, for example, suggest that the click-echoes heard by these patients were processed by brain regions devoted to vision rather than audition.[79]
ADHD stimulants
Reviews of MRI studies on individuals with ADHD suggest that the long-term treatment of attention deficit hyperactivity disorder (ADHD) with stimulants, such as amphetamine or methylphenidate, decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.[80][81][82]
In children
Neuroplasticity is most active in childhood as a part of normal human development, and can also be seen as an especially important mechanism for children in terms of risk and resiliency.[83] Trauma is considered a great risk as it negatively affects many areas of the brain and puts a strain on the sympathetic nervous system from constant activation. Trauma thus alters the brain's connections such that children who have experienced trauma may be hyper vigilant or overly aroused.[84] However a child's brain can cope with these adverse effects through the actions of neuroplasticity.[85]
There are many examples of neuroplasticity in human development. In an article written by Justine Ker and Stephen Nelson, the effects of musical training on neuroplasticity is looked at. Musical training is a form of experience dependent plasticity. This is when changes in the brain occur based on experiences that are unique to an individual. Examples of this are learning multiple languages, playing a sport, doing theatre, etc. A study done by Hyde in 2009, showed that changes in the brain of children could be seen in as little as 15 months of musical training.[86] Ker and Nelson suggest this degree of plasticity in the brain's of children can "help provide a form of intervention for children... with developmental disorders and neurological diseases."[87]
In animals
In a single lifespan, individuals of an animal species may encounter various changes in brain morphology. Many of these differences are caused by the release of hormones in the brain; others are the product of evolutionary factors or developmental stages.[88][89][90][91] Some changes occur seasonally in species to enhance or generate response behaviors.
Seasonal brain changes
Changing brain behavior and morphology to suit other seasonal behaviors is relatively common in animals.[92] These changes can improve the chances of mating during breeding season.[88][89][90][92][93][94] Examples of seasonal brain morphology change can be found within many classes and species.
Within the class Aves, black-capped chickadees experience an increase in the volume of their hippocampus and strength of neural connections to the hippocampus during fall months.[95][96] These morphological changes within the hippocampus which are related to spatial memory are not limited to birds, as they can also be observed in rodents and amphibians.[92] In songbirds, many song control nuclei in the brain increase in size during mating season.[92] Among birds, changes in brain morphology to influence song patterns, frequency, and volume are common.[97] Gonadotropin-releasing hormone (GnRH) immunoreactivity, or the reception of the hormone, is lowered in European starlings exposed to longer periods of light during the day.[88][89]
The California sea hare, a gastropod, has more successful inhibition of egg-laying hormones outside of mating season due to increased effectiveness of inhibitors in the brain.[90] Changes to the inhibitory nature of regions of the brain can also be found in humans and other mammals.[91] In the amphibian Bufo japonicus, part of the amygdala is larger before breeding and during hibernation than it is after breeding.[93]
Seasonal brain variation occurs within many mammals. Part of the hypothalamus of the common ewe is more receptive to GnRH during breeding season than at other times of the year.[94] Humans experience a change in the "size of the hypothalamic suprachiasmatic nucleus and vasopressin-immunoreactive neurons within it"[91] during the fall, when these parts are larger. In the spring, both reduce in size.[98]
Traumatic brain injury research
Randy Nudo's group found that if a small stroke (an infarction) is induced by obstruction of blood flow to a portion of a monkey's motor cortex, the part of the body that responds by movement moves when areas adjacent to the damaged brain area are stimulated. In one study, intracortical microstimulation (ICMS) mapping techniques were used in nine normal monkeys. Some underwent ischemic-infarction procedures and the others, ICMS procedures. The monkeys with ischemic infarctions retained more finger flexion during food retrieval and after several months this deficit returned to preoperative levels.[99] With respect to the distal forelimb representation, "postinfarction mapping procedures revealed that movement representations underwent reorganization throughout the adjacent, undamaged cortex."[99] Understanding of interaction between the damaged and undamaged areas provides a basis for better treatment plans in stroke patients. Current research includes the tracking of changes that occur in the motor areas of the cerebral cortex as a result of a stroke. Thus, events that occur in the reorganization process of the brain can be ascertained. Nudo is also involved in studying the treatment plans that may enhance recovery from strokes, such as physiotherapy, pharmacotherapy, and electrical-stimulation therapy.
Jon Kaas, a professor at Vanderbilt University, has been able to show "how somatosensory area 3b and ventroposterior (VP) nucleus of the thalamus are affected by longstanding unilateral dorsal-column lesions at cervical levels in macaque monkeys."[100] Adult brains have the ability to change as a result of injury but the extent of the reorganization depends on the extent of the injury. His recent research focuses on the somatosensory system, which involves a sense of the body and its movements using many senses. Usually, damage of the somatosensory cortex results in impairment of the body perception. Kaas' research project is focused on how these systems (somatosensory, cognitive, motor systems) respond with plastic changes resulting from injury.[100]
One recent study of neuroplasticity involves work done by a team of doctors and researchers at Emory University, specifically Dr. Donald Stein[101] and Dr. David Wright. This is the first treatment in 40 years that has significant results in treating traumatic brain injuries while also incurring no known side effects and being cheap to administer.[42] Dr. Stein noticed that female mice seemed to recover from brain injuries better than male mice, and that at certain points in the estrus cycle, females recovered even better. This difference may be attributed to different levels of progesterone, with higher levels of progesterone leading to the faster recovery from brain injury in mice. However, clinical trials showed progesterone offers no significant benefit for traumatic brain injury human patients.[102]
Aging
Transcriptional profiling of the frontal cortex of persons ranging from 26 to 106 years of age defined a set of genes with reduced expression after age 40, and especially after age 70.[103] Genes that play central roles in synaptic plasticity were the most significantly affected by age, generally showing reduced expression over time. There was also a marked increase in cortical DNA damage, likely oxidative DNA damage, in gene promoters with aging.[103]
Reactive oxygen species appear to have a significant role in the regulation of synaptic plasticity and cognitive function.[104] However age-related increases in reactive oxygen species may also lead to impairments in these functions.
Multilingualism
The beneficial effect of multilingualism on people's behavior and cognition is well-known nowadays. Numerous studies have shown that people who study more than one language have better cognitive functions and flexibilities than people who only speak one language. Bilinguals are found to have longer attention spans, stronger organization and analyzation skills, and a better theory of mind than monolinguals. Researchers have found that the effect of multilingualism on better cognition is due to neuroplasticity.
In one prominent study, neurolinguists used a voxel-based morphometry (VBM) method to visualize the structural plasticity of brains in healthy monolinguals and bilinguals. They first investigated the differences in density of grey and white matter between two groups and found the relationship between brain structure and age of language acquisition. The results showed that grey-matter density in the inferior parietal cortex for multilingual individuals were significantly greater than monolingual individuals. The researchers also found that early bilinguals had a greater density of grey matter relative to late bilinguals in the same region. The inferior parietal cortex is a brain region highly associated with the language learning, which corresponds to the VMB result of the study.[105]
Recent studies have also found that learning multiple languages not only re-structures the brain but also boosts brain's capacity for plasticity. A recent study found that multilingualism not only affects the grey matter but also white matter of the brain. White matter is made up of myelinated axons that is greatly associated with learning and communication. Neurolinguists used a diffusion tensor imaging (DTI) scanning method to determine the white matter intensity between monolinguals and bilinguals. Increased myelinations in white matter tracts were found in bilingual individuals who actively use the both languages in everyday life. The demand of handling more than one language requires more efficient connectivity within the brain and grey matter, which resulted in greater white matter density for multilinguals.[106]
While it is still debated whether these changes in brain are result of genetic disposition or environmental demands, many evidences suggest that environmental, social experience in early multilinguals affect the structural and functional reorganization in the brain.[107][108]
See also
- Activity-dependent plasticity
- Environmental enrichment (neural)
- Neural backpropagation
- Neuroplastic effects of pollution
- Kinesiology
- Lumosity
References
- Duffau, Hugues (2016). "Brain Plasticity and Reorganization Before, During, and After Glioma Resection". Glioblastoma. pp. 225–236. doi:10.1016/B978-0-323-47660-7.00018-5. ISBN 978-0-323-47660-7.
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR (1964). "Chemical and Anatomical Plasticity of the Brain". Science. 146 (3644): 610–619. Bibcode:1964Sci...146..610B. doi:10.1126/science.146.3644.610. PMID 14191699.
- Livingston R.B. (1966). "Brain mechanisms in conditioning and learning". Neurosciences Research Program Bulletin. 4 (3): 349–354.
- Rakic, P. (January 2002). "Neurogenesis in adult primate neocortex: an evaluation of the evidence". Nature Reviews Neuroscience. 3 (1): 65–71. doi:10.1038/nrn700. PMID 11823806.
- Sasmita, Andrew Octavian; Kuruvilla, Joshua; Ling, Anna Pick Kiong (4 May 2018). "Harnessing neuroplasticity: modern approaches and clinical future". The International Journal of Neuroscience. 128 (11): 1061–1077. doi:10.1080/00207454.2018.1466781. ISSN 1563-5279. PMID 29667473.
- Hensch, Takao K.; Bilimoria, Parizad M. (29 August 2012). "Re-opening Windows: Manipulating Critical Periods for Brain Development". Cerebrum: The Dana Forum on Brain Science. 2012: 11. PMC 3574806. PMID 23447797.
- Ryugo, D. K. and Limb C. J. (2000)."Brain Plastivity: The impact of the environment on the brain as it relates to hearing and deafness" In Cochlear Implants: Principles & Practices. Dordrecht: Kluwer Academic Publishers.
- Pascual-Leone A.; Freitas C.; Oberman L.; Horvath J. C.; Halko M.; Eldaief M.; et al. (2011). "Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI". Brain Topography. 24 (3–4): 302–315. doi:10.1007/s10548-011-0196-8. PMC 3374641. PMID 21842407.
- Ganguly K, Poo MM (October 2013). "Activity-dependent neural plasticity from bench to bedside". Neuron. 80 (3): 729–741. doi:10.1016/j.neuron.2013.10.028. PMID 24183023.
- Keller TA, Just MA (January 2016). "Structural and functional neuroplasticity in human learning of spatial routes". NeuroImage. 125: 256–266. doi:10.1016/j.neuroimage.2015.10.015. PMID 26477660.
Recent findings with both animals and humans suggest that decreases in microscopic movements of water in the hippocampus reflect short-term neuroplasticity resulting from learning. Here we examine whether such neuroplastic structural changes concurrently alter the functional connectivity between hippocampus and other regions involved in learning. ... These concurrent changes characterize the multidimensionality of neuroplasticity as it enables human spatial learning.
- "The Principles of Psychology", William James 1890, Chapter IV, Habits
- LeDoux, Joseph E. (2002). Synaptic self: how our brains become who we are. New York, United States: Viking. p. 137. ISBN 978-0-670-03028-6.
- Rosenzweig, Mark R. (1996). "Aspects of the search for neural mechanisms of memory". Annual Review of Psychology. 47: 1–32. doi:10.1146/annurev.psych.47.1.1. PMID 8624134.
- Meghan O'Rourke Train Your Brain 25 April 2007
- Mateos-Aparicio, Pedro; Rodriguez-Moreno, Antonio (2019). "The Impact of Studying Brain Plasticity". Frontiers in Cellular Neuroscience. 13 (66): 66. doi:10.3389/fncel.2019.00066. PMC 6400842. PMID 30873009.
- Fuchs, Eberhard; Flügge, Gabriele (2014). "Adult neuroplasticity: more than 40 years of research". Neural Plasticity. 2014 (5): 541870. doi:10.1155/2014/541870. PMC 4026979. PMID 24883212.
- Shaw, Christopher; McEachern, Jill, eds. (2001). Toward a theory of neuroplasticity. London, England: Psychology Press. ISBN 978-1-84169-021-6.
- Gonzalo, J. (1945, 1950, 1952, 2010). Dinámica Cerebral. Facsimil edition of Volumen I 1945 and Volumen II 1950 (Madrid: Inst. S. Ramón y Cajal, CSIC), Suplemento I 1952 (Trab. Inst. Cajal Invest. Biol.), first ed. Suplemento II 2010. Santiago de Compostela, Spain: Red Temática en Tecnologías de Computación Artificial/Natural (RTNAC) and Universidad de Santiago de Compostela (USC). ISBN 978-84-9887-458-7. Open Access. For a recent review in English see this article (Open Access).English translation of: Article of 1952 and Indexes of Vol. I (1945) and Vol. II (1950), Open Access.
- Stratton G.M. (1896). "Some preliminary experiments on vision without inversion of the retinal image". Psychological Review. 3 (6): 611–7. doi:10.1037/h0072918.
- Gonzalo, J. (1952). "Las funciones cerebrales humanas según nuevos datos y bases fisiológicas. Una introducción a los estudios de Dinámica Cerebral". Trabajos del Inst. Cajal de Investigaciones Biológicas XLIV: pp. 95–157. [Facsimil edition as `Splemento I´ in Dinámica Cerebral (2010), Open Access. Complete English translation, Open Access.
- Diamond MC, Krech D, Rosenzweig MR (1964). "The Effects of an Enriched Environment on the Histology of the Rat Cerebral Cortex". J Comp Neurol. 123: 111–120. doi:10.1002/cne.901230110. PMID 14199261.
- Brain Science Podcast Episode #10, "Neuroplasticity"
- Doidge, Norman (2007). The Brain That Changes Itself: Stories of Personal Triumph from the frontiers of brain science. New York: Viking. ISBN 978-0-670-03830-5.
- "Wired Science . Video: Mixed Feelings". PBS. Retrieved 12 June 2010.
- "Shepherd Ivory Franz". Rkthomas.myweb.uga.edu. Archived from the original on 3 February 2012. Retrieved 12 June 2010.
- Colotla, Victor A.; Bach-y-Rita, Paul (2002). "Shepherd Ivory Franz: His contributions to neuropsychology and rehabilitation" (PDF). Cognitive, Affective & Behavioral Neuroscience. 2 (2): 141–148. doi:10.3758/CABN.2.2.141. PMID 12455681. Archived from the original on 1 March 2012.CS1 maint: unfit url (link)
- Maguire, E. A.; Frackowiak, R. S.; Frith, C. D. (1997). "Recalling routes around london: Activation of the right hippocampus in taxi drivers". The Journal of Neuroscience. 17 (18): 7103–7110. doi:10.1523/JNEUROSCI.17-18-07103.1997. PMC 6573257. PMID 9278544.
- Woollett, K.; Maguire, E. A. (2011). "Acquiring "the Knowledge" of London's Layout Drives Structural Brain Changes". Current Biology. 21 (24): 2109–2114. doi:10.1016/j.cub.2011.11.018. PMC 3268356. PMID 22169537.
- Maguire, E. A.; Gadian, D. G.; Johnsrude, I. S.; Good, C. D.; Ashburner, J.; Frackowiak, R. S. J.; Frith, C. D. (2000). "Navigation-related structural change in the hippocampi of taxi drivers". Proceedings of the National Academy of Sciences. 97 (8): 4398–4403. Bibcode:2000PNAS...97.4398M. doi:10.1073/pnas.070039597. PMC 18253. PMID 10716738.
- "2016 Kavli Prize in Neuroscience". 2 June 2016.
- Wall, J.T.; Xu, J.; Wang, X. (September 2002). "Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body". Brain Research Reviews. 39 (2–3): 181–215. doi:10.1016/S0165-0173(02)00192-3. PMID 12423766.
- Zilles, Karl (October 1992). "Neuronal plasticity as an adaptive property of the central nervous system". Annals of Anatomy - Anatomischer Anzeiger. 174 (5): 383–91. doi:10.1016/s0940-9602(11)80255-4. PMID 1333175.
- Chang, Yongmin (2014). "Reorganization and plastic changes of the human brain associated with skill learning and expertise". Frontiers in Human Neuroscience. 8 (55): 35. doi:10.3389/fnhum.2014.00035. PMC 3912552. PMID 24550812.
- Mateos-Aparicio, Pedro; Rodriguez-Moreno, Antonio (2019). "The Impact of Studying Brain Plasticity". Frontiers in Cellular Neuroscience. 13 (66): 66. doi:10.3389/fncel.2019.00066. PMC 6400842. PMID 30873009.
- Freed, William J; De Medinaceli, Luis; Wyatt, Richard J (1985). "Promoting functional plasticity in the damaged nervous system". Science. 227 (4694): 1544–52. Bibcode:1985Sci...227.1544F. doi:10.1126/science.3975624. PMID 3975624.
- Patten, Anna R; Yau, Suk Yu; Fontaine, Christine J; Meconi, Alicia; Wortman, Ryan C; Christie, Brian R (2015). "The benefits of exercise on structural and functional plasticity in the rodent hippocampus of different disease models". Brain Plasticity. 1 (1): 97–127. doi:10.3233/BPL-150016. PMC 5928528. PMID 29765836.
- Ponti, Giovanna; Peretto, Paolo; Bonfanti, Luca; Reh, Thomas A. (2008). Reh, Thomas A. (ed.). "Genesis of Neuronal and Glial Progenitors in the Cerebellar Cortex of Peripuberal and Adult Rabbits". PLoS ONE. 3 (6): e2366. Bibcode:2008PLoSO...3.2366P. doi:10.1371/journal.pone.0002366. PMC 2396292. PMID 18523645.
- França, Thiago (2018). "Plasticity and redundancy in the integration of adult born neurons in the hippocampus". Neurobiology of Learning and Memory. 155: 136–142. doi:10.1016/j.nlm.2018.07.007. PMID 30031119.
- Young J. A., Tolentino M.; Tolentino (2011). "Neuroplasticity and its Applications for Rehabilitation". American Journal of Therapeutics. 18 (1): 70–80. doi:10.1097/MJT.0b013e3181e0f1a4. PMID 21192249.
- Traumatic Brain Injury (a story of TBI and the results of ProTECT using progesterone treatments) Emory University News Archives
- Cutler, Sarah M.; Hoffman, Stuart W.; Pettus, Edward H.; Stein, Donald G. (October 2005). "Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury". Experimental Neurology. 195 (2): 423–429. doi:10.1016/j.expneurol.2005.06.003. PMID 16039652.
- Stein, Donald. "Plasticity." Personal interview. Alyssa Walz. 19 November 2008.
- Progesterone offers no significant benefit in traumatic brain injury clinical trial, Emory University, Atlanta, GA
- Dominick M. Maino: Neuroplasticity: Teaching an Old Brain New Tricks, Review of Optometry, January 2009
- Indu Vedamurthy; Samuel J. Huang; Dennis M. Levi; Daphne Bavelier; David C. Knill (27 December 2012). "Recovery of stereopsis in adults through training in a virtual reality task". Journal of Vision. 12 (14). doi:10.1167/12.14.53. Article 53
- Robert F. Hess; Benjamin Thompson (February 2013). "New insights into amblyopia: binocular therapy and noninvasive brain stimulation". Journal of AAPOS. 17 (1). pp. 89–93. doi:10.1016/j.jaapos.2012.10.018.
- Strong GK, Torgerson CJ, Torgerson D, Hulme C (March 2011). "A systematic meta-analytic review of evidence for the effectiveness of the 'Fast ForWord' language intervention program". J Child Psychol Psychiatry. 52 (3): 224–35. doi:10.1111/j.1469-7610.2010.02329.x. PMC 3061204. PMID 20950285.
- Scientific Learning (2011). "In Response to a Meta-Analysis by Strong et al" (PDF). Scientific Learning. Retrieved 16 September 2019.
- Kral A, Sharma A; Sharma (2012). "Developmental Neuroplasticity after Cochlear Implantation". Trends Neurosci. 35 (2): 111–122. doi:10.1016/j.tins.2011.09.004. PMC 3561718. PMID 22104561.
- Kral A, O'Donoghue GM (2010). "Profound Deafness in Childhood". New England Journal of Medicine. 363 (15): 1438–50. doi:10.1056/nejmra0911225. PMID 20925546.
- Beaumont, Geneviève; Mercier, Pierre-Emmanuel; Malouin, Jackson (2011). "Decreasing phantom limb pain through observation of action and imagery: A case series". Pain Medicine. 12 (2): 289–299. doi:10.1111/j.1526-4637.2010.01048.x. PMID 21276185.
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N; Elbert; Knecht; Wienbruch; Pantev; Birbaumer; Larbig; Taub; et al. (1995). "Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation". Nature. 375 (6531): 482–484. Bibcode:1995Natur.375..482F. doi:10.1038/375482a0. PMID 7777055.CS1 maint: multiple names: authors list (link)
- Flor H, Cortical Reorganization And Chronic Pain: Implications For Rehabilitation, J Rehabil Med, 2003, Suppl.41:66–72
- Moseley, Brugger, Interdependence of movement and anatomy persists when amputees learn a physiologically impossible movement of their phantom limb, PNAS, 16 September 2009,
- Seifert F.; Maihöfner C. (2011). "Functional and structural imaging of pain-induced neuroplasticity". Current Opinion in Anesthesiology. 24 (5): 515–523. doi:10.1097/aco.0b013e32834a1079. PMID 21822136.
- Maihöfner C.; Handwerker H.O.; Neundorfer B.; Birklein F. (2003). "Patterns of cortical reorganization in complex regional pain syndrome". Neurology. 61 (12): 1707–1715. doi:10.1212/01.wnl.0000098939.02752.8e. PMID 14694034.
- Apkarian A.V., Sosa Y., Sonty S; Sosa; Sonty; Levy; Harden; Parrish; Gitelman; et al. (2004). "Chronic back pain is associated with decreased prefrontal and thalamic gray matter density". J Neurosci. 24 (46): 10410–10415. doi:10.1523/JNEUROSCI.2541-04.2004. PMC 6730296. PMID 15548656.CS1 maint: multiple names: authors list (link)
- Karl A., Birbaumer N., Lutzenberger W.; Birbaumer; Lutzenberger; Cohen; Flor; et al. (2001). "Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain". J Neurosci. 21 (10): 3609–18. doi:10.1523/JNEUROSCI.21-10-03609.2001. PMC 6762494. PMID 11331390.CS1 maint: multiple names: authors list (link)
- Flor H.; Braun C.; Elbert T.; et al. (1997). "Extensive reorganization of primary somatosensory cortex in chronic back pain patients". Neurosci Lett. 224 (1): 5–8. doi:10.1016/s0304-3940(97)13441-3. PMID 9132689.
- Napadow V., Kettner N., Ryan A.; Kettner; Ryan; Kwong; Audette; Hui; et al. (2006). "Somatosensory cortical plasticity in carpal tunnel syndrome: a cross-sectional fMRI evaluation". NeuroImage. 31 (2): 520–530. doi:10.1016/j.neuroimage.2005.12.017. PMID 16460960.CS1 maint: multiple names: authors list (link)
- Pagnoni, Giuseppe; Cekic, Milos (28 July 2007). "Age effects on gray matter volume and attentional performance in Zen meditation". Neurobiology of Aging. 28 (10): 1623–1627. doi:10.1016/j.neurobiolaging.2007.06.008. hdl:11380/609140. PMID 17655980.
- Vestergaard-Poulsen, Peter; van Beek, Martijn; Skewes, Joshua; Bjarkam, Carsten R; Stubberup, Michael; Bertelsen, Jes; Roepstorff, Andreas (28 January 2009). "Long-term meditation is associated with increased gray matter density in the brain stem". NeuroReport. 20 (2): 170–174. doi:10.1097/WNR.0b013e328320012a. PMID 19104459.
- Luders, Eileen; Toga, Arthur W.; Lepore, Natasha; Gaser, Christian (14 January 2009). "The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter". NeuroImage. 45 (3): 672–678. doi:10.1016/j.neuroimage.2008.12.061. PMC 3184843. PMID 19280691.
- Lazar, S.; Kerr, C.; Wasserman, R.; Gray, J.; Greve, D.; Treadway, Michael T.; McGarvey, Metta; Quinn, Brian T.; et al. (28 November 2005). "Meditation experience is associated with increased cortical thickness". NeuroReport. 16 (17): 1893–97. doi:10.1097/01.wnr.0000186598.66243.19. PMC 1361002. PMID 16272874.
- Lutz, A.; Greischar, L.L.; Rawlings, N.B.; Ricard, M.; Davidson, R. J. (16 November 2004). "Long-term meditators self-induce high-amplitude gamma synchrony during mental practice". PNAS. 101 (46): 16369–73. Bibcode:2004PNAS..10116369L. doi:10.1073/pnas.0407401101. PMC 526201. PMID 15534199.
- Sharon Begley (20 January 2007). "How Thinking Can Change the Brain". dalailama.com. Archived from the original on 9 May 2007. Retrieved 10 May 2007.
- Davidson, Richard; Lutz, Antoine (January 2008). "Buddha's Brain: Neuroplasticity and Meditation" (PDF). IEEE Signal Processing Magazine. Archived (PDF) from the original on 12 January 2012. Retrieved 19 April 2018.
- Chris Frith (17 February 2007). "Stop meditating, start interacting". New Scientist.
- Tarumi T, Zhang R (January 2014). "Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise". Front Physiol. 5: 6. doi:10.3389/fphys.2014.00006. PMC 3896879. PMID 24478719.
Exercise-related improvements in brain function and structure may be conferred by the concurrent adaptations in vascular function and structure. Aerobic exercise increases the peripheral levels of growth factors (e.g., BDNF, IFG-1, and VEGF) that cross the blood-brain barrier (BBB) and stimulate neurogenesis and angiogenesis (Trejo et al., 2001; Lee et al., 2002; Fabel et al., 2003; Lopez-Lopez et al., 2004).
- Szuhany KL, Bugatti M, Otto MW (October 2014). "A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor". J Psychiatr Res. 60C: 56–64. doi:10.1016/j.jpsychires.2014.10.003. PMC 4314337. PMID 25455510.
Consistent evidence indicates that exercise improves cognition and mood, with preliminary evidence suggesting that brain-derived neurotrophic factor (BDNF) may mediate these effects. The aim of the current meta-analysis was to provide an estimate of the strength of the association between exercise and increased BDNF levels in humans across multiple exercise paradigms. We conducted a meta-analysis of 29 studies (N = 1111 participants) examining the effect of exercise on BDNF levels in three exercise paradigms: (1) a single session of exercise, (2) a session of exercise following a program of regular exercise, and (3) resting BDNF levels following a program of regular exercise. Moderators of this effect were also examined. Results demonstrated a moderate effect size for increases in BDNF following a single session of exercise (Hedges' g = 0.46, p < 0.001). Further, regular exercise intensified the effect of a session of exercise on BDNF levels (Hedges' g = 0.59, p = 0.02). Finally, results indicated a small effect of regular exercise on resting BDNF levels (Hedges' g = 0.27, p = 0.005). ... Effect size analysis supports the role of exercise as a strategy for enhancing BDNF activity in humans
- Gomez-Pinilla, Fernando; Hillman, Charles (2013). "The Influence of Exercise on Cognitive Abilities". Comprehensive Physiology. 3. pp. 403–28. doi:10.1002/cphy.c110063. ISBN 9780470650714. PMC 3951958. PMID 23720292.
- Erickson KI, Leckie RL, Weinstein AM (September 2014). "Physical activity, fitness, and gray matter volume". Neurobiol. Aging. 35 Suppl 2: S20–528. doi:10.1016/j.neurobiolaging.2014.03.034. PMC 4094356. PMID 24952993.
- Erickson KI, Miller DL, Roecklein KA (2012). "The aging hippocampus: interactions between exercise, depression, and BDNF". Neuroscientist. 18 (1): 82–97. doi:10.1177/1073858410397054. PMC 3575139. PMID 21531985.
- Lees C, Hopkins J (2013). "Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: a systematic review of randomized control trials". Prev Chronic Dis. 10: E174. doi:10.5888/pcd10.130010. PMC 3809922. PMID 24157077.
- Carvalho A, Rea IM, Parimon T, Cusack BJ (2014). "Physical activity and cognitive function in individuals over 60 years of age: a systematic review". Clin Interv Aging. 9: 661–682. doi:10.2147/CIA.S55520. PMC 3990369. PMID 24748784.
- Guiney H, Machado L (February 2013). "Benefits of regular aerobic exercise for executive functioning in healthy populations". Psychon Bull Rev. 20 (1): 73–86. doi:10.3758/s13423-012-0345-4. PMID 23229442.
- Buckley J, Cohen JD, Kramer AF, McAuley E, Mullen SP (2014). "Cognitive control in the self-regulation of physical activity and sedentary behavior". Front Hum Neurosci. 8: 747. doi:10.3389/fnhum.2014.00747. PMC 4179677. PMID 25324754.
- Thaler, L.; Arnott, S. R.; Goodale, M. A. (13 August 2010). "Human Echolocation I". Journal of Vision. 10 (7): 1050. doi:10.1167/10.7.1050.
- Thaler L, Arnott SR, Goodale MA (2011). "Neural Correlates of Natural Human Echolocation in Early and Late Blind Echolocation Experts". PLOS ONE. 6 (5): e20162. Bibcode:2011PLoSO...620162T. doi:10.1371/journal.pone.0020162. PMC 3102086. PMID 21633496.
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". J. Clin. Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta Psychiatrica Scand. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- Masten, AS (May 2011). "Resilience in children threatened by extreme adversity: frameworks for research, practice, and translational synergy". Development and Psychopathology. 23 (2): 493–506. doi:10.1017/S0954579411000198. PMID 23786691.
- Schore, Allan N. (2001). "The effects of early relational trauma on right brain development, affect regulation, and infant mental health". Infant Mental Health Journal. 1 (2): 201–269. doi:10.1002/1097-0355(200101/04)22:1<201::AID-IMHJ8>3.0.CO;2-9.
- Cioni, Giovanni; d'Acunto, Giulia; Guzzetta, Andrea (2011). "Perinatal brain damage in children". Gene Expression to Neurobiology and Behavior: Human Brain Development and Developmental Disorders. Progress in Brain Research. 189. pp. 139–154. doi:10.1016/B978-0-444-53884-0.00022-1. ISBN 9780444538840. PMID 21489387.
- Hyde, Krista L.; Lerch, Jason; Norton, Andrea; Forgeard, Marie; Winner, Ellen; Evans, Alan C.; Schlaug, Gottfried (11 March 2009). "Musical Training Shapes Structural Brain Development". Journal of Neuroscience. 29 (10): 3019–3025. doi:10.1523/JNEUROSCI.5118-08.2009. PMID 19279238.
- Ker J, Nelson S (2019) The Effects of Musical Training on Brain Plasticity and Cognitive Processes. Jr Neuro Psych and Brain Res: JNPBR-127.
- Parry D.M.; et al. (1997). "Immunocytochemical localization of GnRH precursor in the hypothalamus of European starlings during sexual maturation and photorefractoriness". J. Neuroendocrinol. 9 (3): 235–243. doi:10.1046/j.1365-2826.1997.00575.x. PMID 9089475.
- D.M. Parry, A.R. Goldsmith Ultrastructural evidence for changes in synaptic input to the hypothalamic luteinizing hormone-releasing hormone neurons in photosensitive and photorefractory starlings J. Neuroendocrinol., 5 (1993), pp. 387–395
- Wayne N.L.; et al. (1998). "Seasonal fluctuations in the secretory response of neuroendocrine cells of Aplysia californica to inhibitors of protein kinase A and protein kinase C". Gen. Comp. Endocrinol. 109 (3): 356–365. doi:10.1006/gcen.1997.7040. PMID 9480743.
- M.A. Hofman, D.F. Swaab "Seasonal changes in the suprachiasmatic nucleus of man Neurosci. Lett. 1992; 139, pp. 257–260
- F. Nottebohm A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain Science, 214 (1981), pp. 1368–1370
- Takami S.; Urano A. (1984). "The volume of the toad medial amygdala-anterior preoptic complex is sexually dimorphic and seasonally variable". Neurosci. Lett. 44 (3): 253–258. doi:10.1016/0304-3940(84)90031-4. PMID 6728295.
- J.J. Xiong et al. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: Changes in synaptic inputs onto GnRH neurons Endocrinology, 138 (1997), pp. 1240–1250
- Barnea A.; Nottebohm F. (1994). "Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees". Proc. Natl. Acad. Sci. U.S.A. 91 (23): 11217–11221. Bibcode:1994PNAS...9111217B. doi:10.1073/pnas.91.23.11217. PMC 45198. PMID 7972037.
- Smulders T.V.; et al. (1995). "Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee". J. Neurobiol. 27 (1): 15–25. doi:10.1002/neu.480270103. PMID 7643072.
- Smith G.T. (1996). "Seasonal plasticity in the song nuclei of wild rufous-sided towhees". Brain Res. 734 (1–2): 79–85. doi:10.1016/0006-8993(96)00613-0. PMID 8896811.
- Anthony D. Tramontin, Eliot A. Brenowitz "Seasonal plasticity in the adult brain. Trends in Neurosciences, Volume 23, Issue 6, 1 June 2000, Pages 251–258
- Frost, S.B.; Barbay, S.; Friel, K.M.; Plautz, E.J.; Nudo, R.J. (2003). "Reorganization of Remote Cortical Regions After Ischemic Brain Injury: A Potential Substrate for Stroke Recovery". Journal of Neurophysiology. 89 (6): 3205–3214. doi:10.1152/jn.01143.2002. PMID 12783955.
- Jain, Neeraj; Qi, HX; Collins, CE; Kaas, JH (22 October 2008). "Large-Scale Reorganization in the Somatosensory Cortex and Thalamus after Sensory Loss in Macaque Monkeys". The Journal of Neuroscience. 28 (43): 11042–11060. doi:10.1523/JNEUROSCI.2334-08.2008. PMC 2613515. PMID 18945912.
- "Coulter Department of Biomedical Engineering: BME Faculty". Bme.gatech.edu. Archived from the original on 24 June 2008. Retrieved 12 June 2010.
- "Progesterone offers no significant benefit in traumatic brain injury clinical trial". news.emory.edu. 10 December 2014. Retrieved 29 December 2016.
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (June 2004). "Gene regulation and DNA damage in the ageing human brain". Nature. 429 (6994): 883–91. Bibcode:2004Natur.429..883L. doi:10.1038/nature02661. PMID 15190254.
- Massaad CA, Klann E (May 2011). "Reactive oxygen species in the regulation of synaptic plasticity and memory". Antioxid. Redox Signal. 14 (10): 2013–54. doi:10.1089/ars.2010.3208. PMC 3078504. PMID 20649473.
- Mechelli, Andrea; Crinion, Jenny T; Noppeney, Uta; O'Doherty, John; Ashburner, John; Frackowiak, Richard S; Price, Cathy J (2004). "Neurolinguistics: structural plasticity in the bilingual brain". Nature. 431 (7010): 757. Bibcode:2004Natur.431..757M. doi:10.1038/431757a. PMID 15483594.
- Pliatsikas, Christos; Moschopoulou, Elisavet; Saddy, James D (2015). "The effects of bilingualism on the white matter structure of the brain". Proceedings of the National Academy of Sciences. 112 (5): 1334–7. doi:10.1073/pnas.1414183112. PMC 4321232. PMID 25583505.
- Draganski, Bogdan; Gaser, Christian; Busch, Volker; Schuierer, Gerhard; Bogdahn, Ulrich; May, Arne (2004). "Neuroplasticity: changes in grey matter induced by training". Nature. 427 (6972): 311–2. Bibcode:2004Natur.427..311D. doi:10.1038/427311a. PMID 14737157.
- Golestani, Narly; Paus, Tomáš; Zatorre, Robert J (2002). "Anatomical correlates of learning novel speech sounds". Neuron. 35 (5): 997–1010. doi:10.1016/S0896-6273(02)00862-0. PMID 12372292.
- Buonomano, Dean V.; Merzenich, Michael M. (March 1998). "CORTICAL PLASTICITY: From Synapses to Maps". Annual Review of Neuroscience. 21: 149–186. doi:10.1146/annurev.neuro.21.1.149. PMID 9530495.
- Merzenich, M.M.; Nelson, R.J.; Stryker, M.P.; Cynader, M.S.; Schoppmann, A.; Zook, J.M. (1984). "Somatosensory Cortical Map Changes Following Digit Amputation in Adult Monkeys". Journal of Comparative Neurology. 224 (4): 591–605. doi:10.1002/cne.902240408. PMID 6725633.
Further reading
- Edelman, Gerald. Bright Air, Brilliant Fire: On the Matter of the Mind (Basic Books, 1992, Reprint edition 1993). ISBN 0-465-00764-3
- Edelman and Jean-Pierre Changeux, editors, The Brain (Transaction Publishers, 2000).
- Pinaud, Raphael; Tremere, Liisa A.; De Weerd, Peter, eds. (2006). Plasticity in the visual system: from genes to circuits. New York: Springer. ISBN 978-0-387-28190-2.
- Pinaud, Raphael; Tremere, Liisa A., eds. (2006). Immediate early genes in sensory processing, cognitive performance and neurological disorders. New York: Springer. ISBN 978-0-387-33603-9.
- Begley, Sharon (5 November 2004). "Scans of Monks' Brains Show Meditation Alters Structure, Functioning". The Wall Street Journal. Washington D.C. p. B1. Archived from the original on 2 February 2008.
- Donoghue, John P. (2002). "Connecting cortex to machines: recent advances in brain interfaces" (PDF). Nature Neuroscience. 5: 1085–1088. doi:10.1038/nn947. PMID 12403992. Archived from the original (PDF) on 20 July 2011. Retrieved 1 February 2010.
- Flor, H. (July 2002). "Phantom-limb pain: characteristics, causes, and treatment". The Lancet Neurology. 1 (3): 182–189. doi:10.1016/S1474-4422(02)00074-1. PMID 12849487.
- Ramachandran, Vilayanur S.; Hirstein, William (1998). "The perception of phantom limbs. The D. O. Hebb lecture". Brain. 121 (9): 1603–1630. doi:10.1093/brain/121.9.1603. PMID 9762952.
- Cohen, Wendy; Hodson, Ann; O'Hare, Anne; Boyle, James; Durrani, Tariq; McCartney, Elspeth; Mattey, Mike; Naftalin, Lionel; Watson, Jocelynne (June 2005). "Effects of Computer-Based Intervention Through Acoustically Modified Speech (Fast ForWord) in Severe Mixed Receptive-Expressive Language Impairment: Outcomes From a Randomized Controlled Trial". Journal of Speech, Language, and Hearing Research. 48 (3): 715–729. doi:10.1044/1092-4388(2005/049). PMID 16197283.
- Giszter, Simon F. (January 2008). "SCI: Present and Future Therapeutic Devices and Prostheses". Neurotherapeutics. 5 (1): 147–162. doi:10.1016/j.nurt.2007.10.062. PMC 2390875. PMID 18164494.
- Mahncke, Henry W.; Connor, Bonnie B.; Appelman, Jed; Ahsanuddin, Omar N.; Hardy, Joseph L.; Wood, Richard A.; Joyce, Nicholas M.; Boniske, Tania; et al. (15 August 2006). "Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study". Proceedings of the National Academy of Sciences of the United States of America. 103 (33): 12523–12528. Bibcode:2006PNAS..10312523M. doi:10.1073/pnas.0605194103. PMC 1526649. PMID 16888038.
- Stein, Donald G.; Hoffman, Stuart W. (July–August 2003). "Concepts of CNS Plasticity in the Context of Brain Damage and Repair". Journal of Head Trauma Rehabilitation. 18 (4): 317–341. doi:10.1097/00001199-200307000-00004. PMID 16222128.
- Nudo, Randolph J.; Milliken, Garrett W. (1996). "Reorganization of Movement Representations in Primary Motor Cortex Following Focal Ischemic Infarct in Adult Squirrel Monkeys". Journal of Neurophysiology. 75 (5): 2144–149. doi:10.1152/jn.1996.75.5.2144. PMID 8734610.
- Fine, Cordelia; Jordan-Young, Rebecca; Kaiser, Anelis; Rippon, Gina (November 2013). "Plasticity, plasticity, plasticity…and the problem of sex" (PDF). Trends in Cognitive Sciences. 17 (11): 550–551. doi:10.1016/j.tics.2013.08.010. PMID 24176517.
- Wieloch, Tadeusz; Nikolich, Karoly (June 2006). "Mechanisms of neural plasticity following brain injury". Current Opinion in Neurobiology. 16 (3): 258–264. doi:10.1016/j.conb.2006.05.011. PMID 16713245.
- Videos
- Ramachandran. Phantom Limb Syndrome. about consciousness, mirror neurons, and phantom limb syndrome
- Other readings
- Chorost, Michael (2005). Rebuilt: how becoming part computer made me more human. Boston: Houghton Mifflin. ISBN 978-0-618-37829-6.
External links
- Neuroplasticity at the US National Library of Medicine Medical Subject Headings (MeSH)
- Neuro Myths: Separating Fact and Fiction in Brain-Based Learning by Sara Bernard