Cochlear implant
A cochlear implant (CI) is a surgically implanted neuroprosthetic device to provide a person with moderate to profound sensorineural hearing loss a modified sense of sound. CI bypasses the normal acoustic hearing process to replace it with electric signals which directly stimulate the auditory nerve. Cochlear implants with intensive auditory training a person may learn to interpret those signals as sound and speech. However, one third of deaf children do not develop language if they are on a CI program alone and have no sign language input.
| Cochlear implant | |
|---|---|
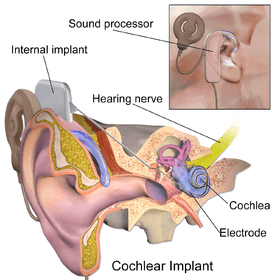 Cochlear implant | |
| MedlinePlus | 007203 |
The implant has two main components. The outside component is generally worn behind the ear, but could also be attached to clothing, for example, in young children. This component, the sound processor, contains microphones, electronics that include Digital Signal Processor chips, battery, and a coil which transmits a signal to the implant across the skin. The inside component, the actual implant, has a coil to receive signals, electronics, and an array of electrodes which is placed into the cochlea, which stimulate the cochlear nerve.
The surgical procedure is performed under general anesthesia. Surgical risks are minimal but can include tinnitus and dizziness.
From the early days of implants in the 1970s and the 1980s, speech perception via an implant has steadily increased. Many users of modern implants gain reasonable to good hearing and speech perception skills post-implantation, especially when combined with lipreading.[1][2] However, for pre-lingually Deaf children the risk of not acquiring spoken language even with an implant may be as high as 30%. One of the challenges that remain with these implants is that hearing and speech understanding skills after implantation show a wide range of variation across individual implant users. Factors such as duration and cause of hearing loss, how the implant is situated in the cochlea, the overall health of the cochlear nerve, but also individual capabilities of re-learning are considered to contribute to this variation, yet no certain predictive factors are known.[3][4][5]
Despite providing the ability for hearing and oral speech communication to children and adults with severe to profound hearing loss, there is also controversy around the devices. Much of the strongest objection to cochlear implants has come from the Deaf community. For some in the Deaf community, cochlear implants are an affront to their culture, which as some view it, is a minority threatened by the hearing majority.[6]
History
André Djourno and Charles Eyriès invented the original cochlear implant in 1957. This original design distributed stimulation using a single channel. Two years later they went their separate ways due to personal and professional differences.[7]
William House also invented a cochlear implant in 1961.[8] In 1964, Blair Simmons and Robert J. White implanted a single-channel electrode in a patient's cochlea at Stanford University.[9]
However, research indicated that these single-channel cochlear implants were of limited usefulness because they can not stimulate different areas of the cochlea at different times to allow differentiation between low and mid to high frequencies as required for detecting speech.[10]
NASA engineer Adam Kissiah started working in the mid-1970s on what could become the modern cochlear implant. Kissiah used his knowledge learned while working as an electronics instrumentation engineer at NASA. This work took place over 3 years, when Kissiah would spend his lunch breaks and evenings in Kennedy’s technical library, studying the impact of engineering principles on the inner ear. In 1977, NASA helped Kissiah obtain a patent for the cochlear implant; Kissiah later sold the patent rights.[11]
The modern multi-channel cochlear implant was independently developed and commercialized by two separate teams—one led by Graeme Clark in Australia and another by Ingeborg Hochmair and her future husband, Erwin Hochmair in Austria, with the Hochmairs' device first implanted in a person in December 1977 and Clark's in August 1978.[12]
Parts
Cochlear implants bypass most of the peripheral auditory system which receives sound and converts that sound into movements of hair cells in the cochlea; the inside-portion of these hair cells release potassium ions in response to the movement of the hairs, and the potassium in turn stimulates other cells to release the neurotransmitter, glutamate, which makes the cochlear nerve send signals to the brain, which creates the experience of sound. Instead, the devices pick up sound and digitize it, convert that digitized sound into electrical signals, and transmit those signals to electrodes embedded in the cochlea. The electrodes electrically stimulate the cochlear nerve, causing it to send signals to the brain.[13][14][15]
There are several systems available, but generally they have the following components:[13][15]
External:
- one or more microphones that pick up sound from the environment
- a speech processor which selectively filters sound to prioritize audible speech
- a transmitter that sends power and the processed sound signals across the skin to the internal device by radio frequency transmission
Internal:
- a receiver/stimulator, which receives signals from the speech processor and converts them into electric impulses
- an electrode array embedded in the cochlea
Surgical procedure
The surgical procedure most often used to implant the device is called mastoidectomy with facial recess approach (MFRA).[15] If a person's individual anatomy prevents MFRA, other approaches, such as going through the suprameatal triangle, are used. A systematic literature review published in 2016 found that studies comparing the two approaches were generally small, not randomized, and retrospective so were not useful for making generalizations; it is not known which approach is safer or more effective.[16]
The procedure is usually done under general anesthesia. Risks of the procedures include mastoiditis, otitis media (acute or with effusion), shifting of the implanted device requiring a second procedure, damage to the facial nerve, damage to the chorda tympani, and wound infections.[16]
The rate of complications is about 12% for minor complications and 3% for major complications; major complications include infections, facial paralysis, and device failure. To avoid the risk of bacterial meningitis, which while low is about thirty times as high compared to people who don't undergo CI procedures, the FDA recommends vaccination prior to the procedure. The rate of transient facial nerve palsy is estimated to be approximately 1%. Device failure requiring reimplantation is estimated to occur in 2.5–6% of the time. Up to one-third of people experience disequilibrium, vertigo, or vestibular weakness lasting more than 1 week after the procedure; in people under 70 these symptoms generally resolve over weeks to months, but in people over 70 the problems tend to persist.[15]
In the past cochlear implants were only approved for people who were deaf in both ears; as of 2014 a cochlear implant had been used experimentally in some people who had acquired deafness in one ear after they had learned how to speak, and none who were deaf in one ear from birth; clinical studies as of 2014 had been too small to draw generalizations from.[17]
Efficacy
A 2011 AHRQ review of the evidence of the effectiveness of CI in people with bilateral hearing loss — the device's primary use — found low to moderate quality data that showed speech perception in noisy conditions was much better for people who had implants in both ears done at the same time compared to people who had only one. The data also showed that no conclusions could be drawn about changes in speech perception in quiet conditions and health-related quality-of-life. There was only one good study comparing implanting implants in both ears at the same time to implanting them sequentially; this study found that in the sequential approach, the second implantation made no change, or made things worse.[18]
Several 2010 and 2012 reviews found that the ability to communicate in spoken language was better the earlier the implantation was performed. The reviews also found that, overall, while cochlear implants provide open set speech understanding for the majority of implanted profoundly hearing-impaired children, the efficacy of cochlear implants is highly variable, and that it was not possible to accurately predict the specific outcome of the given implanted child.[19][20][21]
A 2015 review examined whether CI implantation to treat people with bilateral hearing loss had any effect on tinnitus. This review found the quality of evidence to be poor and the results variable: overall total tinnitus suppression rates varied from 8% to 45% of people who received CI; decrease of tinnitus was seen in 25% to 72%, of people; for 0% to 36% of the people there was no change; increase of tinnitus occurred in between 0% to 25% of patients; and, in between 0 and 10% of cases, people who did not have tinnitus before the procedure, got it.[22]
A 2015 literature review on the use of CI for people with auditory neuropathy spectrum disorder found that, as of that date, description and diagnosis of the condition was too heterogeneous to make clear claims about whether CI is a safe and effective way to manage it.[23]
A 2016 research study found that age at implantation was highly correlated with post-operative speech understanding performance for various test measures. In this study, patients who were implanted at age 65 or older performed significantly worse on speech perception testing in quiet and in noisy conditions compared to younger CI users. The deleterious effects of aging on central auditory processing abilities are thought to play an important role in impacting an individual's speech perception abilities with CI. Prolonged duration of deafness is another factor which is thought to have a negative impact on overall speech understanding outcomes for CI users. However, this particular study found no statistical difference in the speech understanding abilities of CI patients over 65 who had been hearing impaired for 30 years or more prior to implantation.[24] In general, outcomes for CI patients are dependent upon the individual's level of motivation, expectations, exposure to speech stimuli and consistent participation in aural rehabilitation programs.
A 2016 systematic review of CI for people with unilateral hearing loss (UHL) found that of the studies conducted and published, none were randomized, only one evaluated a control group, and no study was blinded. After eliminating multiple uses of the same subjects, the authors found that 137 people with UHL had received a CI.[25] While acknowledging the weakness of the data, the authors found that CI in people with UHL improves sound localization compared with other treatments in people who lost hearing after they learned to speak; in the one study that examined this, CI did improve sound localization in people with UHL who lost hearing before learning to speak.[25] It appeared to improve speech perception and to reduce tinnitus.[25]
Society and culture
Usage
As of October 2010, approximately 188,000 individuals had been fitted with cochlear implants.[26] As of December 2012, the same publication cited approximately 324,000 cochlear implant devices having been surgically implanted. In the U.S., roughly 58,000 devices were implanted in adults and 38,000 in children.[14] As of 2016, the Ear Foundation in the United Kingdom, estimates the number of cochlear implant recipients to be around 600,000.[27]
Cost
In the United States, the overall cost of getting cochlear implants was about $100,000 as of 2017.[28] Some or all of this may be covered by health insurance. In the United Kingdom, the NHS covers cochlear implants in full, as does Medicare in Australia, and the Department of Health[29] in Ireland, Seguridad Social in Spain and Israel, and the Ministry of Health or ACC (depending on the cause of deafness) in New Zealand. According to the US National Institute on Deafness and Other Communication Disorders, the estimated total cost is $60,000 per person implanted.
A study by Johns Hopkins University determined that for a three-year-old child who receives them, cochlear implants can save $30,000 to $50,000 in special-education costs for elementary and secondary schools as the child is more likely to be mainstreamed in school and thus use fewer support services than similarly deaf children.[30]
Manufacturers
As of 2013, the three cochlear implant devices approved for use in the US were manufactured by Cochlear Limited (Australia), Advanced Bionics (a division of Sonova) and MED-EL (Austria). In Europe, Africa, Asia, South America, and Canada, an additional device manufactured by Neurelec (France, a division of William Demant) was available. A device made by Nurotron (China) was also available in some parts of the world. Each manufacturer has adapted some of the successful innovations of the other companies to its own devices. There is no consensus that any one of these implants is superior to the others. Users of all devices report a wide range of performance after implantation.
Criticism and controversy
Much of the strongest objection to cochlear implants has come from within the Deaf community, some of whom are pre-lingually Deaf people whose first language is a sign language. For some in the Deaf community, cochlear implants are an affront to their culture, which, as they view it, is a minority threatened by the hearing majority.[6] This is an old problem for the Deaf community, going back as far as the 18th century with the argument of manualism vs. oralism. This is consistent with medicalisation and the standardisation of the "normal" body in the 19th century, when differences between normal and abnormal began to be debated.[31] It is important to consider the sociocultural context, particularly in regards to the Deaf community, which considers itself to possess its own unique language and culture.[32] This accounts for the cochlear implant being seen as an affront to their culture, as many do not believe that deafness is something that needs to be cured. However, it has also been argued that this does not necessarily have to be the case: the cochlear implant can act as a tool deaf people can use to access the "hearing world" without losing their Deaf identity.[32]
It is believed by some that cochlear implants for congenitally deaf children are most effective when implanted at a young age.[33] However evidence shows that Deaf children who sign well do better academically. Thus specialists recommend that all Deaf children should learn sign language from birth . Deaf culture critics also point out that the cochlear implant and the subsequent therapy often become the focus of the child's identity at the expense of language acquisition and ease of communication in sign language and Deaf identity. They believe that measuring a child's success only by their mastery of speech will lead to a poor self-image as "disabled" (because the implants do not produce normal hearing) rather than having the healthy self-concept of a proudly Deaf person.[34]
Children with cochlear implants are more likely to be educated orally, in the standard fashion, and without access to sign language and are often isolated from other deaf children and from sign language.[35] Cochlear implants have been one of the technological and social factors implicated in the decline of sign languages in the developed world.[36] Some Deaf activists have labeled the widespread implantation of children as "cultural genocide".[37]
As the trend for cochlear implants in children grows, Deaf-community advocates have tried to counter the "either or" formulation of oralism vs manualism with a "both and" approach; some schools are now successfully integrating cochlear implants with sign language in their educational programs.[38]
See also
- Auditory brainstem response
- Auditory brainstem implant
- Bone-anchored hearing aid
- Bone conduction
- Brain implant
- Ear trumpet
- Electric Acoustic Stimulation
- Electrophonic hearing
- Hearing Aid
- Neuroprosthetics
- Noise health effects
- Visual prosthesis
- Language deprivation § Deaf and Hard of Hearing Children
References
- Clark, Graeme M. (April 2015). "The Multi-Channel Cochlear Implant: Multi-Disciplinary Development of Electrical Stimulation of the Cochlea and the Resulting Clinical Benefit". Hearing Research. 322: 4–13. doi:10.1016/j.heares.2014.08.002. PMID 25159273.
- Shannon, Robert V. (February 2012). "Advances in Auditory Prostheses". Current Opinion in Neurology. 25 (1): 61–66. doi:10.1097/WCO.0b013e32834ef878. PMC 4123811. PMID 22157109.
- Blamey, Peter; Artieres, Françoise; Başkent, Deniz; Bergeron, François; Beynon, Andy; Burke, Elaine; Dillier, Norbert; Dowell, Richard; Fraysse, Bernard; Gallégo, Stéphane; Govaerts, Paul J.; Green, Kevin; Huber, Alexander M.; Kleine-Punte, Andrea; Maat, Bert; Marx, Mathieu; Mawman, Deborah; Mosnier, Isabelle; O'Connor, Alec Fitzgerald; O'Leary, Stephen; Rousset, Alexandra; Schauwers, Karen; Skarzynski, Henryk; Skarzynski, Piotr H.; Sterkers, Olivier; Terranti, Assia; Truy, Eric; Van de Heyning, Paul; Venail, Fréderic; Vincent, Christophe; Lazard, Diane S. (2013). "Factors Affecting Auditory Performance of Postlinguistically Deaf Adults Using Cochlear Implants: An Update with 2251 Patients" (PDF). Audiology and Neurotology. 18 (1): 36–47. doi:10.1159/000343189. PMID 23095305.
- Başkent, D.; Gaudrain, E.; Tamati, T.N.; Wagner, A. (2016). Perception and psychoacoustics of speech in cochlear implant users, in Scientific Foundations of Audiology: Perspectives from Physics, Biology, Modeling, and Medicine, Eds. A.T. Cacace, E. de Kleine, A.G. Holt, and P. van Dijk. San Diego, CA, USA: Plural Publishing, Inc. pp. 285–319.
- Pisoni, David B.; Kronenberger, William G.; Harris, Michael S.; Moberly, Aaron C. (December 2017). "Three challenges for future research on cochlear implants". World Journal of Otorhinolaryngology - Head and Neck Surgery. 3 (4): 240–254. doi:10.1016/j.wjorl.2017.12.010. PMC 5956139. PMID 29780970.
- "The Cochlear Implant Controversy, Issues And Debates". NEW YORK: CBS News. September 4, 2001. Retrieved 2008-11-09.
- Svirsky, Mario (2017). "Cochlear implants and electronic hearing". Physics Today. 70 (8): 52–58. Bibcode:2017PhT....70h..52S. doi:10.1063/PT.3.3661. ISSN 0031-9228.
- Martin, Douglas (December 15, 2012). "Dr. William F. House, Inventor of Pioneering Ear-Implant Device, Dies at 89". The New York Times. Retrieved 2012-12-16.
- Mudry, A; Mills, M (May 2013). "The early history of the cochlear implant: a retrospective". JAMA Otolaryngology–Head & Neck Surgery. 139 (5): 446–53. doi:10.1001/jamaoto.2013.293. PMID 23681026.
- Clark, Graeme (2009). "The multi-channel cochlear implant: past, present and future perspectives". Cochlear Implants International. 10 Suppl 1: 2–13. doi:10.1179/cim.2009.10.Supplement-1.2. ISSN 1754-7628. PMID 19127562.
- NASA (2003). NASA space station. Washington, DC: U.S. Government Printing.
- "2013 Lasker~DeBakey Clinical Medical Research Award: Modern cochlear implant". The Lasker Foundation. Retrieved 14 July 2017.
- Roche JP, Hansen MR (2015). "On the Horizon: Cochlear Implant Technology". Otolaryngol. Clin. North Am. 48 (6): 1097–116. doi:10.1016/j.otc.2015.07.009. PMC 4641792. PMID 26443490.
- NIH Publication No. 11-4798 (2013-11-01). "Cochlear Implants". National Institute on Deafness and Other Communication Disorders. Retrieved February 18, 2016.
- Yawn R, Hunter JB, Sweeney AD, Bennett ML (2015). "Cochlear implantation: a biomechanical prosthesis for hearing loss". F1000Prime Rep. 7: 45. doi:10.12703/P7-45. PMC 4447036. PMID 26097718.
- Bruijnzeel H, et al. Systematic Review on Surgical Outcomes and Hearing Preservation for Cochlear Implantation in Children and Adults. Otolaryngol Head Neck Surg. 2016 Feb 16. Review. doi:10.1177/0194599815627146 PMID 26884363
- Tokita, J; Dunn, C; Hansen, MR (October 2014). "Cochlear implantation and single-sided deafness". Current Opinion in Otolaryngology & Head and Neck Surgery. 22 (5): 353–8. doi:10.1097/moo.0000000000000080. PMC 4185341. PMID 25050566.
- Raman G, et al. Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss [Internet]. Agency for Healthcare Research and Quality (US); 2011 Jun 17. PMID 25927131 Free full text
- Kral, Andrej; O'Donoghue, Gerard M. (2010). "Profound Deafness in Childhood". New England Journal of Medicine. 363 (15): 1438–50. doi:10.1056/NEJMra0911225. PMID 20925546.
- Niparko, John K (2010). "Spoken Language Development in Children Following Cochlear Implantation". JAMA. 303 (15): 1498–506. doi:10.1001/jama.2010.451. PMC 3073449. PMID 20407059.
- Ganek, Hillary; Robbins, Amy McConkey; Niparko, John K. (2012). "Language Outcomes After Cochlear Implantation". Otolaryngologic Clinics of North America. 45 (1): 173–185. doi:10.1016/j.otc.2011.08.024. PMID 22115689.
- Ramakers GG, van Zon A, Stegeman I, Grolman W (2015). "The effect of cochlear implantation on tinnitus in patients with bilateral hearing loss: A systematic review". Laryngoscope. 125 (11): 2584–92. doi:10.1002/lary.25370. PMID 26153087.
- Harrison RV, Gordon KA, Papsin BC, Negandhi J, James AL (2015). "Auditory neuropathy spectrum disorder (ANSD) and cochlear implantation". Int. J. Pediatr. Otorhinolaryngol. 79 (12): 1980–7. doi:10.1016/j.ijporl.2015.10.006. PMID 26545793.
- Beyea, Jason A.; McMullen, Kyle P.; Harris, Michael S.; Houston, Derek M.; Martin, Jennifer M.; Bolster, Virginia A.; Adunka, Oliver F.; Moberly, Aaron C. (October 2016). "Cochlear Implants in Adults: Effects of Age and Duration of Deafness on Speech Recognition". Otology & Neurotology. 37 (9): 1238–1245. doi:10.1097/MAO.0000000000001162. ISSN 1537-4505. PMID 27466894.
- Cabral Junior F, Pinna MH, Alves RD, Malerbi AF, Bento RF (2016). "Cochlear Implantation and Single-sided Deafness: A Systematic Review of the Literature". Int Arch Otorhinolaryngol. 20 (1): 69–75. doi:10.1055/s-0035-1559586. PMC 4687988. PMID 26722349.
- "NIH Fact Sheets - Cochlear Implants". report.nih.gov. Retrieved 2018-09-14.
- "Cochlear Implant Information Sheet". The Ear Foundation. Retrieved 2018-09-14.
- "Cochlear Implants". American Academy of Otolaryngology–Head and Neck Surgery. 21 April 2014. Retrieved 12 May 2017.
- "Beaumont Hospital - Cochlear Implant - How to Refer".
- John M. Williams (2000-05-05). "Do Health-Care Providers Have to Pay for Assistive Tech?". Business Week. Retrieved 2009-10-25.
- Lock, M. and Nguyen, V-K., An Anthropology of Biomedicine, Oxford, Wiley-Blackwell, 2010.
- Power D (2005). "Models of deafness: cochlear implants in the Australian daily press". Journal of Deaf Studies and Deaf Education. 10 (4): 451–9. doi:10.1093/deafed/eni042. PMID 16000690.
- Paul Oginni (2009-11-16). "UCI Research with Cochlear Implants No Longer Falling on Deaf Ears". New University. Retrieved 2009-11-18.
- NAD Cochlear Implant Committee. "Cochlear Implants". Archived from the original on 2007-02-20.
- Ringo, Allegra (August 9, 2013). "Understanding Deafness: Not Everyone Wants to Be 'Fixed'". The Atlantic.
- Johnston, T (2004). "W(h)ither the deaf community? Population, genetics, and the future of Australian sign language". American Annals of the Deaf. 148 (5): 358–75. doi:10.1353/aad.2004.0004. PMID 15132016.
- Christiansen, John B.; Leigh, Irene W.; Spencer, Patricia Elizabeth; Lucker, Jay R. (2001). Cochlear implants in children : ethics and choices ([Online-Ausg.] ed.). Washington, D.C.: Gallaudet University Press. pp. 304–305. ISBN 9781563681165.
- Denworth, Lydia (April 25, 2014). "Science Gave My Son the Gift of Sound". Time.
Further reading
- Wilson, Blake S.; Finley, Charles C.; Lawson, Dewey T.; Wolford, Robert D.; Eddington, Donald K.; Rabinowitz, William M. (1991). "Better speech recognition with cochlear implants". Nature. 352 (6332): 236–8. Bibcode:1991Natur.352..236W. doi:10.1038/352236a0. PMID 1857418.
External links
| Wikimedia Commons has media related to Cochlear implants. |
- Cochlear Implants at Curlie
- Cochlear Implants Information from the National Institutes of Health (NIH).
- NASA Spinoff article on engineer Adam Kissiah's contribution to cochlear implants beginning in the 1970s.