Neurogenesis
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSC)s, and it occurs in all species of animals except the porifera (sponges) and placozoans.[1] Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INP)s, subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.[2] Neurogenesis is most active during embryonic development, and is responsible for producing all the various types of neurons of the organism, but continues throughout adult life in a variety of organisms.[3] Once born, neurons do not divide (see mitosis), and many will live the lifetime of the animal.[4]
| Neurogenesis | |
|---|---|
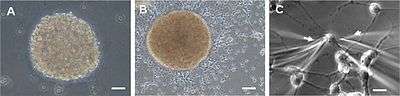 A neurosphere of neural stem cells in rat embryo spreads out into a single layer of cells. A) Neurosphere of subventricular zone cells after two days in culture. B) Shows the neurosphere at four days in culture and cells migrating away. C) Cells at the periphery of the neurosphere mostly having extending processes. | |
| Identifiers | |
| MeSH | D055495 |
| Anatomical terminology | |
Neurogenesis in mammals
Developmental neurogenesis
During embryonic development, the mammalian central nervous system (CNS; brain and spinal cord) is derived from the neural tube, which contains NSCs that will later generate neurons.[5] However, neurogenesis doesn't begin until a sufficient population of NSCs has been achieved. These early stem cells are called neuroepithelial cells (NEC)s, but soon take on a highly elongated radial morphology and are then known as radial glial cells (RGC)s.[6] RGCs are the primary stem cells of the mammalian CNS, and reside in the embryonic ventricular zone, which lies adjacent to the central fluid-filled cavity (ventricular system) of the neural tube.[7][8] Following RGC proliferation, neurogenesis involves a final cell division of the parent RGC, which produces one of two possible outcomes. First, this may generate a subclass of neuronal progenitors called intermediate neuronal precursors (INP)s, which will divide one or more additional times to produce neurons. Alternatively, daughter neurons may be produced directly. Neurons do not immediately form neural circuits through the growth of axons and dendrites. Instead, newborn neurons must first migrate long distances to their final destinations, maturing and finally generating neural circuitry. For example, neurons born in the ventricular zone migrate radially to the cortical plate, which is where neurons accumulate to form the cerebral cortex.[9][10] Thus, the generation of neurons occurs in a specific tissue compartment or 'neurogenic niche' occupied by their parent stem cells.
The rate of neurogenesis and the type of neuron generated (broadly, excitatory or inhibitory) are principally determined by molecular and genetic factors. These factors notably include the Notch signaling pathway, and many genes have been linked to Notch pathway regulation.[11][12] The genes and mechanisms involved in regulating neurogenesis are the subject of intensive research in academic, pharmaceutical, and government settings worldwide.
The amount of time required to generate all the neurons of the CNS varies widely across mammals, and brain neurogenesis is not always complete by the time of birth.[13] For example, mice undergo cortical neurogenesis from about embryonic day (post-conceptional day) (E)11 to E17, and are born at about E19.5.[14] Ferrets are born at E42, although their period of cortical neurogenesis does not end until a few days after birth.[15] In contrast, neurogenesis in humans generally begins around gestational week (GW) 10 and ends around GW 25 with birth about GW 38-40.[16]
Epigenetic modification
As embryonic development of the mammalian brain unfolds, neural progenitor and stem cells switch from proliferative divisions to differentiative divisions. This progression leads to the generation of neurons and glia that populate cortical layers. Epigenetic modifications play a key role in regulating gene expression in the cellular differentiation of neural stem cells. Epigenetic modifications include DNA cytosine methylation to form 5-methylcytosine and 5-methylcytosine demethylation.[17][18] These modifications are critical for cell fate determination in the developing and adult mammalian brain.
DNA cytosine methylation is catalyzed by DNA methyltransferases (DNMTs). Methylcytosine demethylation is catalyzed in several stages by TET enzymes that carry out oxidative reactions (e.g. 5-methylcytosine to 5-hydroxymethylcytosine) and enzymes of the DNA base excision repair (BER) pathway.[17]
Adult neurogenesis
Adult neurogenesis has been shown to occur at low levels compared with development, and in only two regions of the brain: the adult subventricular zone (SVZ) of the lateral ventricles, and the dentate gyrus of the hippocampus.[19][20][21]
Subventricular zone
In many mammals, including for example rodents, the olfactory bulb is a brain region containing cells that detect smell, featuring integration of adult-born neurons, which migrate from the SVZ of the striatum to the olfactory bulb through the rostral migratory stream (RMS).[22][23] The migrating neuroblasts in the olfactory bulb become interneurons that help the brain communicate with these sensory cells. The majority of those interneurons are inhibitory granule cells, but a small number are periglomerular cells. In the adult SVZ, the primary neural stem cells are SVZ astrocytes rather than RGCs. Most of these adult neural stem cells lie dormant in the adult, but in response to certain signals, these dormant cells, or B cells, go through a series of stages, first producing proliferating cells, or C cells. The C cells then produce neuroblasts, or A cells, that will become neurons.[24]
Hippocampus
Significant neurogenesis also occurs during adulthood in the hippocampus of many mammals, from rodents to some primates, although its existence in adult humans is debated.[25][26] The hippocampus plays a crucial role in the formation of new declarative memories, and it has been theorized that the reason human infants cannot form declarative memories is because they are still undergoing extensive neurogenesis in the hippocampus and their memory-generating circuits are immature.[27] Many environmental factors, such as exercise, stress, and antidepressants have been reported to change the rate of neurogenesis within the hippocampus of rodents.[28][29] Some evidence indicates postnatal neurogenesis in the human hippocampus decreases sharply in newborns for the first year or two after birth, dropping to "undetectable levels in adults."[30]
Neurogenesis in other organisms
Neurogenesis has been best characterized in the fruit fly, Drosophila melanogaster.[31] In Drosophila, Notch signaling was first described, controlling a cell-to-cell signaling process called lateral inhibition, in which neurons are selectively generated from epithelial cells.[32][33] In some vertebrates, regenerative neurogenesis has also been shown to occur.[34]
See also
- Neurulation
- Gliogenesis
- Noogenesis
References
- Eric R. Kandel, ed. (2006). Principles of neural science (5. ed.). Appleton and Lange: McGraw Hill. ISBN 978-0071390118.
- Eric R. Kandel, ed. (2006). Principles of neural science (5. ed.). Appleton and Lange: McGraw Hill. ISBN 978-0071390118.
- Eric R. Kandel, ed. (2006). Principles of neural science (5. ed.). Appleton and Lange: McGraw Hill. ISBN 978-0071390118.
- Gilbert, Scott F.; College, Swarthmore; Helsinki, the University of (2014). Developmental biology (Tenth ed.). Sunderland, Mass.: Sinauer. ISBN 978-0878939787.
- Gilbert, Scott F.; College, Swarthmore; Helsinki, the University of (2014). Developmental biology (Tenth ed.). Sunderland, Mass.: Sinauer. ISBN 978-0878939787.
- Gilbert, Scott F.; College, Swarthmore; Helsinki, the University of (2014). Developmental biology (Tenth ed.). Sunderland, Mass.: Sinauer. ISBN 978-0878939787.
- Rakic, P (October 2009). "Evolution of the neocortex: a perspective from developmental biology". Nature Reviews. Neuroscience. 10 (10): 724–35. doi:10.1038/nrn2719. PMC 2913577. PMID 19763105.
- Lui, JH; Hansen, DV; Kriegstein, AR (8 July 2011). "Development and evolution of the human neocortex". Cell. 146 (1): 18–36. doi:10.1016/j.cell.2011.06.030. PMC 3610574. PMID 21729779.
- Rakic, P (October 2009). "Evolution of the neocortex: a perspective from developmental biology". Nature Reviews. Neuroscience. 10 (10): 724–35. doi:10.1038/nrn2719. PMC 2913577. PMID 19763105.
- Lui, JH; Hansen, DV; Kriegstein, AR (8 July 2011). "Development and evolution of the human neocortex". Cell. 146 (1): 18–36. doi:10.1016/j.cell.2011.06.030. PMC 3610574. PMID 21729779.
- Kageyama, R; Ohtsuka, T; Shimojo, H; Imayoshi, I (November 2008). "Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition". Nature Neuroscience. 11 (11): 1247–51. doi:10.1038/nn.2208. PMID 18956012.
- Rash, BG; Lim, HD; Breunig, JJ; Vaccarino, FM (26 October 2011). "FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis". The Journal of Neuroscience. 31 (43): 15604–17. doi:10.1523/jneurosci.4439-11.2011. PMC 3235689. PMID 22031906.
- Gilbert, Scott F.; College, Swarthmore; Helsinki, the University of (2014). Developmental biology (Tenth ed.). Sunderland, Mass.: Sinauer. ISBN 978-0878939787.
- Abbott, David M. Jacobowitz, Louise C. (1998). Chemoarchitectonic atlas of the developing mouse brain. Boca Raton: CRC Press. ISBN 9780849326677.
- Kroenke, CD; Bayly, PV (24 January 2018). "How Forces Fold the Cerebral Cortex". The Journal of Neuroscience. 38 (4): 767–775. doi:10.1523/JNEUROSCI.1105-17.2017. PMC 5783962. PMID 29367287.
- Malik, S; Vinukonda, G; Vose, LR; Diamond, D; Bhimavarapu, BB; Hu, F; Zia, MT; Hevner, R; Zecevic, N; Ballabh, P (9 January 2013). "Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth". The Journal of Neuroscience. 33 (2): 411–23. doi:10.1523/JNEUROSCI.4445-12.2013. PMC 3711635. PMID 23303921.
- Wang Z, Tang B, He Y, Jin P. DNA methylation dynamics in neurogenesis. Epigenomics. 2016 Mar;8(3):401-14. doi: 10.2217/epi.15.119. Epub 2016 Mar 7. Review. PMID:26950681
- Noack F, Pataskar A, Schneider M, Buchholz F, Tiwari VK, Calegari F. Assessment and site-specific manipulation of DNA (hydroxy-)methylation during mouse corticogenesis. Life Sci Alliance. 2019 Feb 27;2(2). pii: e201900331. doi: 10.26508/lsa.201900331. Print 2019 Apr. PMID:30814272
- Ernst, A; Alkass, K; Bernard, S; Salehpour, M; Perl, S; Tisdale, J; Possnert, G; Druid, H; Frisén, J (27 February 2014). "Neurogenesis in the striatum of the adult human brain". Cell. 156 (5): 1072–83. doi:10.1016/j.cell.2014.01.044. PMID 24561062.
- Lim, DA; Alvarez-Buylla, A (2 May 2016). "The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis". Cold Spring Harbor Perspectives in Biology. 8 (5): a018820. doi:10.1101/cshperspect.a018820. PMC 4852803. PMID 27048191.
- Alvarez-Buylla, A; Lim, DA (4 March 2004). "For the long run: maintaining germinal niches in the adult brain". Neuron. 41 (5): 683–6. doi:10.1016/S0896-6273(04)00111-4. PMID 15003168.
- Ernst, A; Alkass, K; Bernard, S; Salehpour, M; Perl, S; Tisdale, J; Possnert, G; Druid, H; Frisén, J (27 February 2014). "Neurogenesis in the striatum of the adult human brain". Cell. 156 (5): 1072–83. doi:10.1016/j.cell.2014.01.044. PMID 24561062.
- Ming, GL; Song, H (May 26, 2011). "Adult neurogenesis in the mammalian brain: significant answers and significant questions". Neuron. 70 (4): 687–702. doi:10.1016/j.neuron.2011.05.001. PMC 3106107. PMID 21609825.
- Lim, DA; Alvarez-Buylla, A (2 May 2016). "The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis". Cold Spring Harbor Perspectives in Biology. 8 (5): a018820. doi:10.1101/cshperspect.a018820. PMC 4852803. PMID 27048191.
- Sorrells, SF; Paredes, MF; Cebrian-Silla, A; Sandoval, K; Qi, D; Kelley, KW; James, D; Mayer, S; Chang, J; Auguste, KI; Chang, EF; Gutierrez, AJ; Kriegstein, AR; Mathern, GW; Oldham, MC; Huang, EJ; Garcia-Verdugo, JM; Yang, Z; Alvarez-Buylla, A (15 March 2018). "Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults". Nature. 555 (7696): 377–381. Bibcode:2018Natur.555..377S. doi:10.1038/nature25975. PMC 6179355. PMID 29513649.
- Boldrini, M; Fulmore, CA; Tartt, AN; Simeon, LR; Pavlova, I; Poposka, V; Rosoklija, GB; Stankov, A; Arango, V; Dwork, AJ; Hen, R; Mann, JJ (5 April 2018). "Human Hippocampal Neurogenesis Persists throughout Aging". Cell Stem Cell. 22 (4): 589–599.e5. doi:10.1016/j.stem.2018.03.015. PMC 5957089. PMID 29625071.
- Josselyn, Sheena A.; Frankland, Paul W. (2012-09-01). "Infantile amnesia: A neurogenic hypothesis". Learning & Memory. 19 (9): 423–433. doi:10.1101/lm.021311.110. ISSN 1072-0502. PMID 22904373.
- Hanson, Nicola D.; Owens, Michael J.; Nemeroff, Charles B. (2011-12-01). "Depression, Antidepressants, and Neurogenesis: A Critical Reappraisal". Neuropsychopharmacology. 36 (13): 2589–2602. doi:10.1038/npp.2011.220. ISSN 0893-133X. PMC 3230505. PMID 21937982.
- Santarelli, Luca; Saxe, Michael; Gross, Cornelius; Surget, Alexandre; Battaglia, Fortunato; Dulawa, Stephanie; Weisstaub, Noelia; Lee, James; Duman, Ronald (2003-08-08). "Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants". Science. 301 (5634): 805–809. Bibcode:2003Sci...301..805S. doi:10.1126/science.1083328. ISSN 0036-8075. PMID 12907793.
- Sorrells, SF; Paredes, MF; Cebrian-Silla, A; Sandoval, K; Qi, D; Kelley, KW; James, D; Mayer, S; Chang, J; Auguste, KI; Chang, EF; Gutierrez, AJ; Kriegstein, AR; Mathern, GW; Oldham, MC; Huang, EJ; Garcia-Verdugo, JM; Yang, Z; Alvarez-Buylla, A (15 March 2018). "Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults". Nature. 555 (7696): 377–381. Bibcode:2018Natur.555..377S. doi:10.1038/nature25975. PMC 6179355. PMID 29513649.
- Homem, Catarina C. F.; Knoblich, Juergen A. (2012-12-01). "Drosophila neuroblasts: a model for stem cell biology". Development. 139 (23): 4297–4310. doi:10.1242/dev.080515. ISSN 1477-9129. PMID 23132240.
- Axelrod, JD (26 October 2010). "Delivering the lateral inhibition punchline: it's all about the timing". Science Signaling. 3 (145): pe38. doi:10.1126/scisignal.3145pe38. PMID 20978236.
- Huang, C; Chan, JA; Schuurmans, C (2014). "Proneural bHLH Genes in Development and Disease". BHLH Transcription Factors in Development and Disease. Current Topics in Developmental Biology. 110. pp. 75–127. doi:10.1016/B978-0-12-405943-6.00002-6. ISBN 9780124059436. PMID 25248474.
- Alunni, A; Bally-Cuif, L (1 March 2016). "A comparative view of regenerative neurogenesis in vertebrates". Development. 143 (5): 741–753. doi:10.1242/dev.122796. PMC 4813331. PMID 26932669.