HIV vaccine
An HIV vaccine may have the purpose of protecting individuals who do not have HIV from being infected with the virus (a preventive vaccine), or treating an HIV-infected person (a therapeutic vaccine). There are two approaches to an HIV vaccine: an active vaccination approach in which a vaccine aims to induce an immune response against HIV; and a passive vaccination approach in which preformed antibodies against HIV are administered.[1]
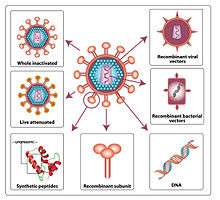
Currently, there is no licensed HIV vaccine on the market, but multiple research projects are trying to find an effective vaccine. There is evidence from humans that a vaccine may be possible. Some, but certainly not all, HIV-infected individuals naturally produce broadly neutralizing antibodies which keep the virus suppressed and these people remain asymptomatic for decades. Potential broadly neutralizing antibodies have been cloned in the laboratory (monoclonal antibodies) and are being tested in passive vaccination clinical trials.
Many trials have shown no efficacy but thus far, one HIV vaccine regimen, RV 144, has been shown to prevent HIV in some individuals in Thailand.
Overview
The urgency of the search for a vaccine against HIV stems from the AIDS-related death toll of over 35 million people since 1981.[2] In 2002, AIDS became the primary cause of death due to an infectious agent in Africa.[3]
Alternative medical treatments to a vaccine exist. For the treatment of HIV-infected individuals, Highly Active Antiretroviral Therapy (HAART) medication has been demonstrated to provide many benefits to HIV-infected individuals, including improved health, increased lifespan, control of viremia, and prevention of transmission to babies and partners. HAART must be taken lifelong without interruption to be effective, and cannot cure HIV. Options for the prevention of HIV infection in HIV-uninfected individuals include safer sex (for example abstinence, partner reduction and condom use), antiretroviral strategies (pre-exposure prophylaxis[4] and post-exposure prophylaxis) and medical male circumcision.[5] Vaccination has proved a powerful public health tool in vanquishing other diseases, and an HIV vaccine is generally considered as the most likely, and perhaps the only way by which the HIV pandemic can be halted. However, HIV-1 remains a challenging target for a vaccine.
Difficulties in developing an HIV vaccine
In 1984, after the confirmation of the etiological agent of AIDS by scientists at the U.S. National Institutes of Health and the Pasteur Institute, the United States Health and Human Services Secretary Margaret Heckler declared that a vaccine would be available within two years.[6] However, the classical vaccination approach that is successful in the control of other viral diseases - priming the adaptive immunity to recognize the viral envelope proteins - have failed to work against HIV. Many factors make the development of an HIV vaccine different from other classic vaccines:[7]
- Classic vaccines mimic natural immunity against reinfection as seen in individuals recovered from infection; there are almost no recovered AIDS patients.
- Most vaccines protect against disease, not against infection; HIV infection may remain latent for long periods before causing AIDS.
- Most effective vaccines are whole-killed or live-attenuated organisms; killed HIV-1 does not retain antigenicity and the use of a live retrovirus vaccine raises safety issues.
HIV structure
The epitopes of the viral envelope are more variable than those of many other viruses. Furthermore, the functionally important epitopes of the gp120 protein are masked by glycosylation, trimerisation and receptor-induced conformational changes making it difficult to block with neutralizing antibodies.
The ineffectiveness of previously developed vaccines primarily stems from two related factors:
- First, HIV is highly mutable. Because of the virus' ability to rapidly respond to selective pressures imposed by the immune system, the population of virus in an infected individual typically evolves so that it can evade the two major arms of the adaptive immune system; humoral (antibody-mediated) and cellular (mediated by T cells) immunity.
- Second, HIV isolates are themselves highly variable. HIV can be categorized into multiple subtypes with a high degree of genetic divergence. Therefore, the immune responses raised by any vaccine need to be broad enough to account for this variability. Any vaccine that lacks this breadth is unlikely to be effective.
The difficulties in stimulating a reliable antibody response has led to the attempts to develop a vaccine that stimulates a response by cytotoxic T-lymphocytes.[8][9]
Another response to the challenge has been to create a single peptide that contains the least variable components of all the known HIV strains.[10]
Animal model
The typical animal model for vaccine research is the monkey, often the macaque. Monkeys can be infected with SIV or the chimeric SHIV for research purposes. However, the well-proven route of trying to induce neutralizing antibodies by vaccination has stalled because of the great difficulty in stimulating antibodies that neutralise heterologous primary HIV isolates.[11] Some vaccines based on the virus envelope have protected chimpanzees or macaques from homologous virus challenge,[12] but in clinical trials, humans who were immunised with similar constructs became infected after later exposure to HIV-1.[13]
There are some differences between SIV and HIV that may introduce challenges in the use of an animal model. The animal model can be extremely useful but at times controversial.[14]
There is a new animal model strongly resembling that of HIV in humans. Generalized immune activation as a direct result of activated CD4+ T cell killing - performed in mice allows new ways of testing HIV behaviour.[15][16]
NIAID-funded SIV research has shown that challenging monkeys with a cytomegalovirus (CMV)-based SIV vaccine results in containment of virus. Typically, virus replication and dissemination occurs within days after infection, whereas vaccine-induced T cell activation and recruitment to sites of viral replication take weeks. Researchers hypothesized that vaccines designed to maintain activated effector memory T cells might impair viral replication at its earliest stage.
Clinical trials to date
Several vaccine candidates are in varying phases of clinical trials.
Phase I
Most initial approaches have focused on the HIV envelope protein. At least thirteen different gp120 and gp160 envelope candidates have been evaluated, in the US predominantly through the AIDS Vaccine Evaluation Group. Most research focused on gp120 rather than gp41/gp160, as the latter is generally more difficult to produce and did not initially offer any clear advantage over gp120 forms. Overall, they have been safe and immunogenic in diverse populations, have induced neutralizing antibody in nearly 100% recipients, but rarely induced CD8+ cytotoxic T lymphocytes (CTL). Mammalian derived envelope preparations have been better inducers of neutralizing antibody than candidates produced in yeast and bacteria. Although the vaccination process involved many repeated "booster" injections, it was challenging to induce and maintain the high anti-gp120 antibody titers necessary to have any hope of neutralizing an HIV exposure.
The availability of several recombinant canarypox vectors has provided interesting results that may prove to be generalizable to other viral vectors. Increasing the complexity of the canarypox vectors by including more genes/epitopes has increased the percent of volunteers that have detectable CTL to a greater extent than did increase the dose of the viral vector. CTLs from volunteers were able to kill peripheral blood mononuclear cells infected with primary isolates of HIV, suggesting that induced CTLs could have biological significance. Besides, cells from at least some volunteers were able to kill cells infected with HIV from other clades, though the pattern of recognition was not uniform among volunteers. The canarypox vector is the first candidate HIV vaccine that has induced cross-clade functional CTL responses. The first phase I trial of the candidate vaccine in Africa was launched early in 1999 with Ugandan volunteers. The study determined the extent to which Ugandan volunteers have CTL that are active against the subtypes of HIV prevalent in Uganda, A and D. In 2015, a Phase I trial called HVTN 100, chaired by two South African researchers Linda-Gail Bekker and Fatima Laher, tested the combination of a canarypox vector and a gp120 protein adapted for the subtype C HIV common in sub-Saharan Africa, and initial results showed that those who received the vaccine regimen produced strong immune responses early on.[17] The regimen is now in advanced phase testing.
Other strategies that have progressed to phase I trials in uninfected persons include peptides, lipopeptides, DNA, an attenuated Salmonella vector, p24, etc. Specifically, candidate vaccines that induce one or more of the following are being sought:
- neutralizing antibodies active against a broad range of HIV primary isolates;
- cytotoxic T cell responses in a vast majority of recipients;
- strong mucosal immune responses.[18]
In 2011, researchers in National Biotech Centre in Madrid unveiled data from the Phase I clinical trial of their new vaccine, MVA-B. The vaccine induced an immunological response in 92% of the healthy subjects.[19]
In 2016, results were published of the first Phase I human clinical trial of a killed whole-HIV-1 vaccine, SAV001. HIV used in the vaccine was chemically and physically deadened through radiation. The trial, conducted in Canada in 2012, demonstrated a good safety profile and elicited antibodies to HIV-1.[20] According to Dr. Chil-Yong Kang of Western University's Schulich School of Medicine & Dentistry in Canada, the developer of this vaccine, antibodies against gp120 and p24 increased to 8-fold and 64-fold, respectively after vaccination.[21]
Phase II
Preventive HIV vaccines
- A recombinant Adenovirus-5 HIV vaccine (called V520) has been tested in two Phase 2b studies, Phambili and STEP. On December 13, 2004, they began recruiting for the STEP study, a 3,000-participant phase II clinical trial of a novel HIV vaccine, at sites in North America, South America, the Caribbean and Australia.[22] The trial was co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), which is a division of the National Institutes of Health (NIH), and the pharmaceutical company Merck & Co. Merck developed V520 to stimulate HIV-specific cellular immunity, which prompts the body to produce T cells that kill HIV-infected cells. In previous smaller trials, this vaccine was found to be safe, because of the lack of adverse effects on the participants. The vaccine showed induced cellular immune responses against HIV in more than half of volunteers.[2] V520 contains a weakened adenovirus that serves as a carrier for three subtype B HIV genes (gag, pol and nef). Subtype B is the most prevalent HIV subtype in the regions of the study sites. Adenoviruses are among the main causes of upper respiratory tract ailments such as the common cold. Because the vaccine contains only three HIV genes housed in a weakened adenovirus, study participants cannot become infected with HIV or get a respiratory infection from the vaccine. It was announced in September 2007 that the trial for V520 would be stopped after it determined that vaccination with V520 appeared associated with an increased risk of HIV infection in some recipients.[23] The foremost issue facing the recombinant adenovirus that was used is the high prevalence of the adenovirus-specific antibodies as a result of prior exposure to adenovirus. Adenovirus vectors and many other viral vectors currently used in HIV vaccines will induce a rapid memory immune response against the vector. This results in an impediment to the development of a T cell response against the inserted antigen (HIV antigens)[24] The results of the trial prompted the reexamination of vaccine development strategies.[25]
- In May 2016, Lawrence Corey and Myron Cohen of the HIV Vaccine Trials Network (HVTN) and HIV Prevention Trials Network (HPTN) respectively partnered to launch the global Antibody Mediated Prevention trial (HVTN 703 and HVTN 704), the first phase IIb trial of a monoclonal antibody for HIV prevention. Monoclonal antibodies (mAbs) are a passive vaccination strategy. HVTN 703 and HVTN 704 test VRC01, a mAb that targets the CD4 binding site.[26]
- In 2017, Janssen and the HVTN launched the phase IIb trial called HVTN 705/Imbokodo, testing the mosaic vector vaccine Ad26.Mos4.HIV and the aluminum phosphate-adjuvanted Clade C gp140 vaccines which are designed to prevent infection of all HIV subtypes around the world.[27]
Therapeutic HIV vaccines
Biosantech developed a therapeutic vaccine called Tat Oyi, which targets the tat protein of HIV. It was tested in France in a double-blind Phase I/II trial with 48 HIV-positive patients who had reached viral suppression on Highly Active Antiretroviral Therapy and then stopped antiretrovirals after getting the intradermal Tat Oyi vaccine.[28]
Phase III
Preventive HIV vaccines
There have been no passive preventive HIV vaccines to reach Phase III yet, but some active preventive HIV vaccine candidates have entered Phase III.
- In February 2003, VaxGen announced that their AIDSVAX B/E vaccine was a failure in North America as there was not a statistically significant reduction of HIV infection within the study population.
- AIDSVAX B/E was a component, along with ALVAC, of the RV 144 vaccine trial in Thailand that showed partial efficacy in preventing HIV. The AIDSVAX B/E and ALVAC vaccines targeted the gp120 part of the HIV envelope. The study involved 16,395 participants who did not have HIV infection, 8197 of whom were given treatment consisting of two experimental vaccines targeting HIV types B and E that are prevalent in Thailand, while 8198 were given a placebo. The participants were tested for HIV every six months for three years. After three years, the vaccine group had HIV infection rates reduced by about 30% compared with those in the placebo group. However, after taking into account the seven people who already had HIV before getting vaccinated (two in the placebo group, five in the vaccine group) the difference was 26%.[29] It was discovered that participants receiving vaccines in the RV 144 trial who produced IgG antibodies against the V2 loop of the HIV outer envelope were 43% less likely to become infected than those who did not, while IgA production was associated with a 54% greater risk of infection than those who did not produce the antibodies (but not worse than placebo). Viruses collected from vaccinated participants had mutations in the V2 region. Tests of a vaccine for SIV in monkeys found greater resistance to SIV in animals producing antibodies against this region. Therefore, further research is expected to focus on creating vaccines designed to provoke an IgG reaction against the V2 loop.[30]
- In 2016, the phase IIb-III trial HVTN 702/"Uhambo" was launched in South Africa. HVTN 702 is testing vaccines similar to those used in RV 144 but adapted for the subtype of HIV common to the region where most new HIV infections occur, sub-Saharan Africa. The trial vaccines are the ALVAC vector vaccine and a two-component gp120 protein subunit vaccine with MF59 adjuvant. HVTN 702, chaired by four South African researchers Glenda Gray, Linda-Gail Bekker, Fatima Laher and Mookho Malahleha, is testing whether the regimen is efficacious at preventing HIV infection in adults.[31] A South African nurse, Sr Bontle Modibedi, was the first person ever to vaccinate with the ALVAC-gp120 subtype C vaccines in all phases of human trials[32].
- In 2019, it was announced that the Ad26.Mos4.HIV plus adjuvanted clade C gp140 vaccine regimen would enter a phase III trial called HVTN 706/"Mosaico". The regimen is a combination of an adenovirus vector vaccine engineered against multiple global strains of HIV, and a protein vaccine.[33]
Therapeutic HIV vaccines
No therapeutic HIV vaccine candidates have reached phase 3 testing yet.
Economics of vaccine development
A July 2012 report of the HIV Vaccines & Microbicides Resource Tracking Working Group estimates that $845 million was invested in HIV vaccine research in 2011.[34]
Economic issues with developing an AIDS vaccine include the need for advance purchase commitment (or advance market commitments) because after an AIDS vaccine has been developed, governments and NGOs may be able to bid the price down to marginal cost.[35]
Classification of all theoretically possible HIV vaccines
Theoretically, any possible HIV vaccine must inhibit or stop the HIV virion replication cycle.[36] The targets of a vaccine could be the following stages of the HIV virion cycle:
- Stage I. Free state
- Stage II. Attachment
- Stage III. Penetration
- Stage IV. Uncoating
- Stage V. Replication
- Stage VI. Assembling
- Stage VII. Releasing
Therefore, the following list comprises the current possible approaches for an HIV vaccine:
Filtering virions from blood (Stage I)
- Biological, chemical and/or physical approaches for removing the HIV virions from the blood.
Approaches to catching the virion (Stage I-III, VI, VII)
- Phagocytosis of the HIV virions.
- Chemical or organically based capture (creation of any skin or additional membrane around the virion) of HIV virions
- Chemical or organic attachments to the virion
Approaches to destroying or damaging the virion or its parts (Stage I-VII)
Here, “damage” means inhibiting or stopping the ability of virion to process any of the Phase II-VII. Here are the different classification of methods:
- By nature of method:
- Physical methods (Stage I-VII)
- Chemical and biological methods (Stage I-VII)
- By damaging target of the HIV virion structure:[37][38]
- Damaging the Docking Glycoprotein gp120[39] (Stage I-III, VI, VII)
- Damaging the Transmembrane Glycoprotein gp41[40] (Stage I-III, VI, VII)
- Damaging the virion matrix (Stage I-III, VI, VII)
- Damaging the virion Capsid (Stage I-III, VI, VII)
- Damaging the Reverse Transcriptase (Stage I-VII)
- Damaging the RNA (Stage I-VII)
Blocking replication (Stage V)
- Insertion into blood chemical or organic compounds which binds to the gp120. Hypothetically, it can be pieces of the CD4 cell membranes with receptors. Any chemical and organic alternative (with the ability to bind the gp120) of these receptors also can be used.
- Insertion into blood chemical or organic compounds which binds to the receptors of the CD4 cells.
Biological, chemical or physical approaches to inhibit the process of phases
- Biological, chemical or physical approach to inhibit the Attachment, Penetration, Uncoating, Integration, Replication, Assembling and/or Releasing.
Inhibiting the functionality of infected cells (Stage VI-VII)
Inhibiting the life functions of infected cells:
- Inhibiting the metabolism of infected cells
- Inhibiting the energy exchange of infected cells
Future work
There have been reports that HIV patients coinfected with GB virus C (GBV-C), also called hepatitis G virus, can survive longer than those without GBV-C, but the patients may be different in other ways. GBV-C is potentially useful in the future development of an HIV vaccine.[41]
Live attenuated vaccines are highly successful against polio, rotavirus and measles, but has not been tested against HIV in humans. Reversion to live virus has been a theoretical safety concern that has to date prevented clinical development of a live attenuated HIV-1 vaccine. Scientists are researching novel strategies to develop a non-virulent live attenuated HIV-1 vaccine. For example, a genetically modified form of HIV has been created in which the virus' codons (a sequence of three nucleotides that form genetic code) are manipulated to rely on an unnatural amino acid for proper protein translation, which allows it to replicate. Because this amino acid is foreign to the human body, the virus cannot reproduce.[42]
Scientists at Scripps Research found a way to attach HIV-fighting antibodies to immune cells, creating an HIV-resistant cell population.[43]
See also
- HIV Vaccine Trials Network
- World AIDS Vaccine Day
References
- Gray GE, Laher F, Lazarus E, Ensoli B, Corey L (April 2016). "Approaches to preventative and therapeutic HIV vaccines". Current Opinion in Virology. 17: 104–109. doi:10.1016/j.coviro.2016.02.010. PMC 5020417. PMID 26985884.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) (December 2005). "AIDS epidemic update" (PDF). World Health Organization. Retrieved 2014-04-22.
- UNAIDS (2004) Report on the global AIDS epidemic, July 2004
- "1 October news". Clinical Infectious Diseases. 55 (7): i–ii. October 2012. doi:10.1093/cid/cis674. PMID 23126012.
- Gray GE, Laher F, Doherty T, Abdool Karim S, Hammer S, Mascola J, Beyrer C, Corey L (March 2016). "Which New Health Technologies Do We Need to Achieve an End to HIV/AIDS?". PLoS Biology. 14 (3): e1002372. doi:10.1371/journal.pbio.1002372. PMC 4774984. PMID 26933962.
- Shilts, Randy (1987). And the Band Played On: Politics, People, and the AIDS Epidemic (2007 ed.). St. Martin's Press. p. 451. ISBN 978-0-312-24135-3.
- Fauci AS (1996). "An HIV vaccine: breaking the paradigms". Proc. Am. Assoc. Phys. 108: 6.
- Kim D, Elizaga M, Duerr A (March 2007). "HIV vaccine efficacy trials: towards the future of HIV prevention". Infectious Disease Clinics of North America. 21 (1): 201–17, x. doi:10.1016/j.idc.2007.01.006. PMID 17502236.
- Watkins DI (March 2008). "The hope for an HIV vaccine based on induction of CD8+ T lymphocytes--a review". Memórias do Instituto Oswaldo Cruz. 103 (2): 119–29. doi:10.1590/S0074-02762008000200001. PMC 2997999. PMID 18425263.
- Létourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, Hanke T (October 2007). Nixon D (ed.). "Design and pre-clinical evaluation of a universal HIV-1 vaccine". PLOS ONE. 2 (10): e984. Bibcode:2007PLoSO...2..984L. doi:10.1371/journal.pone.0000984. PMC 1991584. PMID 17912361.
- Poignard P, Sabbe R, Picchio GR, Wang M, Gulizia RJ, Katinger H, et al. (April 1999). "Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo". Immunity. 10 (4): 431–8. doi:10.1016/S1074-7613(00)80043-6. PMID 10229186.
- Berman PW, Gregory TJ, Riddle L, Nakamura GR, Champe MA, Porter JP, et al. (June 1990). "Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160". Nature. 345 (6276): 622–5. Bibcode:1990Natur.345..622B. doi:10.1038/345622a0. PMID 2190095.
- Connor RI, Korber BT, Graham BS, Hahn BH, Ho DD, Walker BD, et al. (February 1998). "Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines". Journal of Virology. 72 (2): 1552–76. PMC 124637. PMID 9445059.
- Morgan C, Marthas M, Miller C, Duerr A, Cheng-Mayer C, Desrosiers R, et al. (August 2008). "The use of nonhuman primate models in HIV vaccine development". PLoS Medicine. 5 (8): e173. doi:10.1371/journal.pmed.0050173. PMC 2504486. PMID 18700814.
- Marques R, Williams A, Eksmond U, Wullaert A, Killeen N, Pasparakis M, et al. (2009). "Generalized immune activation as a direct result of activated CD4+ T cell killing". Journal of Biology. 8 (10): 93. doi:10.1186/jbiol194. PMC 2790834. PMID 19943952.
- Vrisekoop N, Mandl JN, Germain RN (2009). "Life and death as a T lymphocyte: from immune protection to HIV pathogenesis". Journal of Biology. 8 (10): 91. doi:10.1186/jbiol198. PMC 2790836. PMID 19951397.
- Bekker LG, Moodie Z, Grunenberg N, Laher F, Tomaras GD, Cohen KW, et al. (June 2018). "Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial". The Lancet. HIV. 5 (7): e366–e378. doi:10.1016/S2352-3018(18)30071-7. PMC 6028742. PMID 29898870.
- Pavot V, Rochereau N, Lawrence P, Girard MP, Genin C, Verrier B, Paul S (July 2014). "Recent progress in HIV vaccines inducing mucosal immune responses". AIDS. 28 (12): 1701–18. doi:10.1097/qad.0000000000000308. PMID 25009956.
- "New Vaccine Could Turn HIV Into Minor Infection". Fox News. 2011-09-29. Retrieved 29 September 2011.
- Choi E, Michalski CJ, Choo SH, Kim GN, Banasikowska E, Lee S, et al. (November 2016). "First Phase I human clinical trial of a killed whole-HIV-1 vaccine: demonstration of its safety and enhancement of anti-HIV antibody responses". Retrovirology. 13 (1): 82. doi:10.1186/s12977-016-0317-2. PMC 5126836. PMID 27894306.
- "New HIV Vaccine Proves Successful In Phase 1 Human Trial". Medical Daily. New York. 2013-09-04. Retrieved 2013-09-04.
- "STEP Study Locations". Retrieved 2008-11-04.
- Efficacy Results from the STEP Study (Merck V520 Protocol 023/HVTN 502): A Phase II Test-of-Concept Trial of the MRKAd5 HIV-1 Gag/Pol/Nef Trivalent Vaccine Archived 2011-07-26 at the Wayback Machine
- Sekaly RP (January 2008). "The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development?". The Journal of Experimental Medicine. 205 (1): 7–12. doi:10.1084/jem.20072681. PMC 2234358. PMID 18195078.
- Iaccino E, Schiavone M, Fiume G, Quinto I, Scala G (July 2008). "The aftermath of the Merck's HIV vaccine trial". Retrovirology. 5: 56. doi:10.1186/1742-4690-5-56. PMC 2483718. PMID 18597681.
- Huang Y, Karuna S, Carpp LN, Reeves D, Pegu A, Seaton K, et al. (April 2018). "Modeling cumulative overall prevention efficacy for the VRC01 phase 2b efficacy trials". Human Vaccines & Immunotherapeutics. 14 (9): 2116–2127. doi:10.1080/21645515.2018.1462640. PMC 6183277. PMID 29683765.
- "Candidate for new AIDS vaccine advances to next phase of pre-approval trials- Technology News, Firstpost". Tech2. 2018-07-08. Retrieved 2018-07-11.
- Loret EP, Darque A, Jouve E, Loret EA, Nicolino-Brunet C, Morange S, et al. (April 2016). "Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial". Retrovirology. 13: 21. doi:10.1186/s12977-016-0251-3. PMC 4818470. PMID 27036656.
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. (December 2009). "Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand". The New England Journal of Medicine. 361 (23): 2209–20. doi:10.1056/NEJMoa0908492. PMID 19843557.
- Callaway E (16 September 2011). "Clues emerge to explain first successful HIV vaccine trial". Nature. doi:10.1038/news.2011.541.
- "First new HIV vaccine efficacy study in seven years has begun". National Institutes of Health. 2016-11-28.
- "VOICES - Issue 1 - HIV Vaccine Awareness Day". IAVI - International AIDS Vaccine Initiative. Retrieved 2019-05-25.
- 15 July 2019. "NIH and partners to launch HIV vaccine efficacy trial in the Americas and Europe". National Institutes of Health. Retrieved 23 July 2019.
- "Investing to End the AIDS Epidemic: A new Era for HIV Prevention Research and Development" (PDF). Archived from the original (PDF) on 2012-12-14. Retrieved 2010-12-13.
- Berndt ER, Glennerster R, Kremer M, Lee J, Levine R, Weizsacker G, et al. (April 2005). "Advanced Purchase Commitments for a Malaria Vaccine: Estimating Costs and Effectiveness". doi:10.2139/ssrn.696741. SSRN 696741. Cite journal requires
|journal=(help) - Collier L, Balows A, Sussman M (1998). Mahy B, Collier L (eds.). Virology. Topley and Wilson's Microbiology and Microbial Infections. 1 (ninth ed.). Hodder Education Publishers. pp. 75–91. ISBN 978-0-340-66316-5.
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK (April 2002). "A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening". Journal of Medicinal Chemistry. 45 (8): 1712–22. doi:10.1021/jm010533y. PMID 11931626.
- Compared with overview in: Fisher, Bruce; Harvey, Richard P.; Champe, Pamela C. (2007). Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstown, MD: Lippincott Williams & Wilkins. ISBN 0-7817-8215-5. Page 3
- Foley B, Leitner T, Apetrei C, Hahn B, Mizrachi I, Mullins J, Rambaut A, Wolinsky S, Korber B (2017). HIV Sequence Compendium (Report). Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, NM, LA-UR 17-25240.
- Malashkevich VN, Chan DC, Chutkowski CT, Kim PS (August 1998). "Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9134–9. Bibcode:1998PNAS...95.9134M. doi:10.1073/pnas.95.16.9134. PMC 21304. PMID 9689046.
- Bagasra O, Bagasra AU, Sheraz M, Pace DG (March 2012). "Potential utility of GB virus type C as a preventive vaccine for HIV-1". Expert Review of Vaccines. 11 (3): 335–47. doi:10.1586/erv.11.191. PMID 22380825.
- Wang N, Li Y, Niu W, Sun M, Cerny R, Li Q, Guo J (May 2014). "Construction of a live-attenuated HIV-1 vaccine through genetic code expansion". Angewandte Chemie. 53 (19): 4867–71. doi:10.1002/anie.201402092. PMC 4984542. PMID 24715496.
- "Potential HIV cure with cells resistant to HIV". Medix Frontiers. Retrieved 15 April 2017.
External links
- Vaccine Research Center (VRC)- Information concerning Preventive HIV vaccine research studies
- NIAID HIV vaccine site (DAIDS)
- Global Alliance for Vaccines and Immunization (GAVI)
- International AIDS Vaccine Initiative (IAVI)
- AIDS Vaccine Advocacy Coalition (AVAC)
- U.S. Military HIV Research Program (MHRP)
- Investigation of first candidate vaccine
- Be the Generation - Information on HIV Vaccine Clinical Research in 20 American Cities
- AIDS.gov - The U.S. Federal Domestic HIV/AIDS Resource
- HIVtest.org - Find an HIV testing site near you
- Potential HIV vaccine using adenoviridae vectors
- Bit by Bit, Scientists Gain Ground on AIDS - The New York Times, March 8, 2019