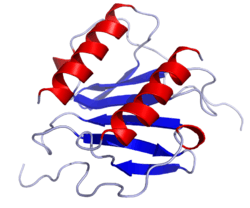
Interleukin 8
Interleukin 8 (IL8 or chemokine (C-X-C motif) ligand 8, CXCL8) is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells[3] and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel-Palade bodies.[4][5] In humans, the interleukin-8 protein is encoded by the CXCL8 gene.[6] IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms.[7] In culture, a 72 amino acid peptide is the major form secreted by macrophages.[7]
There are many receptors on the surface membrane capable of binding IL-8; the most frequently studied types are the G protein-coupled serpentine receptors CXCR1 and CXCR2. Expression and affinity for IL-8 differs between the two receptors (CXCR1 > CXCR2). Through a chain of biochemical reactions, IL-8 is secreted and is an important mediator of the immune reaction in the innate immune system response.
Function
IL-8, also known as neutrophil chemotactic factor, has two primary functions. It induces chemotaxis in target cells, primarily neutrophils but also other granulocytes, causing them to migrate toward the site of infection. IL-8 also stimulates phagocytosis once they have arrived. IL-8 is also known to be a potent promoter of angiogenesis. In target cells, IL-8 induces a series of physiological responses required for migration and phagocytosis, such as increases in intracellular Ca2+, exocytosis (e.g. histamine release), and the respiratory burst.
IL-8 can be secreted by any cells with toll-like receptors that are involved in the innate immune response. Usually, it is the macrophages that see an antigen first, and thus are the first cells to release IL-8 to recruit other cells. Both monomer and homodimer forms of IL-8 have been reported to be potent inducers of the chemokine receptors CXCR1 and CXCR2. The homodimer is more potent, but methylation of Leu25 can block the activity of homodimers.
IL-8 is believed to play a role in the pathogenesis of bronchiolitis, a common respiratory tract disease caused by viral infection.
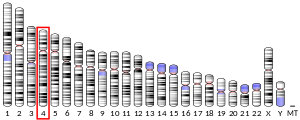
IL-8 is a member of the CXC chemokine family. The genes encoding this and the other ten members of the CXC chemokine family form a cluster in a region mapped to chromosome 4q.[6][8]
CXCL-8 mediated chemotaxis of the neutrophil
CXCL8 is the primary cytokine involved in the recruitment of neutrophils to the site of damage or infection; in a process called chemotaxis. A number of variables are essential for the successful chemotaxis of neutrophils, including the increased expression of high affinity adhesion molecules to secure the neutrophil to the endothelium near the affected site (and is therefore not washed away into the circulatory system), and that the neutrophil can digest its way through the basement membrane and the extracellular matrix (ECM) to reach affected site. CXCL8 plays a key role in inducing the cell signalling necessary to bring about these changes.[9]
Firstly, at the site of infection histamine release causes vasodilation of the capillaries near the injured area which slows down the blood flow in the region and encourages leukocytes, such as neutrophils, to come closer to the endothelium, and away from the centre of the lumen where the rate of blood flow is highest. Once this occurs weak interactions are made between the selectins expressed on the neutrophil and endothelial cells (expression of which is also increased through the action of CXCL8 and other cytokines). On the neutrophil these are: L selectins, and on the endothelial cell: P and E selectins. This causes the "rolling" phase of chemotaxis.
Once the neutrophil is rolling along the endothelium, it will come into contact with a CXCL8 molecule expressed on the surface which stimulates the cell signalling pathway, mediated through a G-coupled-protein-receptor. The binding of CXCL8 to CXCR1/2 on the neutrophil stimulates the neutrophils to upregulate their expression of the integrin, LFA-1, which takes part in high affinity bonding with ICAM-1 receptors expressed on the endothelium. The expression and affinity of LFA-1 is significantly increased to maximise binding. This causes the neutrophil to slow down more until it is stationary. Another key function of the cell signalling stimulated by CXCL8, is the initiation of the oxidative burst. This process allows the build up of proteolytic enzymes and reactive oxygen species (ROS) which are necessary to break down the ECM and basement membrane. These are released in secretory granules, along with more integrins. The release of ROS and damaging enzymes is regulated to minimise host damage, but continues to reach site of infection at which it will carry out its effector functions.[9]
Target cells
While neutrophil granulocytes are the primary target cells of IL-8, there are a relatively wide range of cells (endothelial cells, macrophages, mast cells, and keratinocytes) that respond to this chemokine. The chemoattractant activity of IL-8 in similar concentrations to vertebrates was proven in Tetrahymena pyriformis, which suggests a phylogenetically well-conserved structure and function for this chemokine.[10]
Clinical significance
Interleukin-8 is a key mediator associated with inflammation where it plays a key role in neutrophil recruitment and neutrophil degranulation.[11] As an example, it has been cited as a proinflammatory mediator in gingivitis[12] and psoriasis.
Interleukin-8 secretion is increased by oxidant stress, which thereby cause the recruitment of inflammatory cells and induces a further increase in oxidant stress mediators, making it a key parameter in localized inflammation.[13] IL-8 was shown to be associated with obesity.[14]
IL-8 has also been implied to have a role in colorectal cancer by acting as an autocrine growth factor for colon carcinoma cell lines[15] or the promotion of division and possible migration by cleaving metalloproteinase molecules.[16]
If a pregnant mother has high levels of interleukin-8, there is an increased risk of schizophrenia in her offspring.[17] High levels of Interleukin 8 have been shown to reduce the likelihood of positive responses to antipsychotic medication in schizophrenia.[18]
IL-8 has also been implicated in the pathology of cystic fibrosis. Through its action as a signalling molecule IL-8 is capable of recruiting and guiding neutrophils to the lung epithelium. Overstimulation and dysfunction of these recruited neutrophils within the airways results in release of a number of pro-inflammatory molecules and proteases resulting in further damage of lung tissue.[19]
Nomenclature
IL-8 was renamed CXCL8 by the Chemokine Nomenclature Subcommittee of the International Union of Immunological Societies,.[20] Its approved HUGO gene symbol is CXCL8.
Regulation of expression
The expression of IL-8 is negatively regulated by a number of mechanisms. MiRNA-146a/b-5p indirectly represses IL-8 expression by silencing the expression of IRAK1.[21] Additionally, the 3'UTR of IL-8 contains a A/U-rich element that makes it extremely unstable under certain conditions. IL-8 expression is also regulated by the transcription factor NF-κB.[22] NF-κB regulation represents a novel anti-IL-8 therapy for use in inflammatory diseases such as cystic fibrosis. Pathways leading to the induction of ribosomal protein S6 (rpS6) phosphorylation have also been found to enhance IL-8 protein synthesis. This translational control of IL-8 expression is dependent on A/U-rich proximal sequences (APS), which are found in the 3'UTR of IL-8 immediately after the stop codon.[23]
See also
- Interleukin 8 receptor, alpha
- Interleukin 8 receptor, beta
References
- GRCh38: Ensembl release 89: ENSG00000169429 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Hedges JC, Singer CA, Gerthoffer WT (2000). "Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes". Am. J. Respir. Cell Mol. Biol. 23 (1): 86–94. CiteSeerX 10.1.1.326.6212. doi:10.1165/ajrcmb.23.1.4014. PMID 10873157.
- Wolff B, Burns AR, Middleton J, Rot A (1998). "Endothelial cell "memory" of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies". J. Exp. Med. 188 (9): 1757–62. doi:10.1084/jem.188.9.1757. PMC 2212526. PMID 9802987.
- Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G (1998). "Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells". J. Exp. Med. 188 (9): 1751–6. doi:10.1084/jem.188.9.1751. PMC 2212514. PMID 9802986.
- Modi WS, Dean M, Seuanez HN, Mukaida N, Matsushima K, O'Brien SJ (1990). "Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily". Hum. Genet. 84 (2): 185–7. doi:10.1007/BF00208938. PMID 1967588.
- Brat DJ, Bellail AC, Van Meir EG (2005). "The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis". Neuro-oncology. 7 (2): 122–133. doi:10.1215/s1152851704001061. PMC 1871893. PMID 15831231.
- "Entrez Gene: IL8 interleukin 8".
- Dixit N, Simon SI (2012). "Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest". Frontiers in Immunology. 3: 188. doi:10.3389/fimmu.2012.00188. PMC 3392659. PMID 22787461.
- Köhidai L, Csaba G (1998). "Chemotaxis and chemotactic selection induced with cytokines (IL-8, RANTES and TNF-alpha) in the unicellular Tetrahymena pyriformis". Cytokine. 10 (7): 481–6. doi:10.1006/cyto.1997.0328. PMID 9702410.
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K (Nov 1994). "Essential involvement of interleukin-8 (IL-8) in acute inflammation". Journal of Leukocyte Biology. 56 (5): 559–64. doi:10.1002/jlb.56.5.559. PMID 7964163.
- Haake, SK, Huang, GTJ: Molecular Biology of the host-Microbe Interaction in Periodontal Diseases (Selected Topics). In Newman, Takei, Carranza, editors: Clinical Periodontology, 9th Edition. Philadelphia: W.B.Saunders Co. 2002. page 162.
- Vlahopoulos S, Boldogh I, Casola A, Brasier AR (1999). "Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation". Blood. 94 (6): 1878–89. doi:10.1182/blood.V94.6.1878.418k03_1878_1889. PMID 10477716.
- Sharabiani MT, Vermeulen R, Scoccianti C, Hosnijeh FS, Minelli L, Sacerdote C, Palli D, Krogh V, Tumino R, Chiodini P, Panico S, Vineis P (2011). "Immunologic profile of excessive body weight". Biomarkers. 16 (3): 243–51. doi:10.3109/1354750X.2010.547948. PMID 21506696.
- Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE (2000). "Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro". Cytokine. 12 (1): 78–85. doi:10.1006/cyto.1999.0518. PMID 10623446.
- Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, Kubota H, Mori Y, Ohara H, Nomura T, Higashiyama S, Itoh M (2005). "IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells". Cytokine. 29 (6): 275–82. doi:10.1016/j.cyto.2004.11.005. PMID 15749028.
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES (2004). "Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring". Am J Psychiatry. 161 (5): 889–95. doi:10.1176/appi.ajp.161.5.889. PMID 15121655.
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC (2004). "Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia". J Clin Psychiatry. 65 (7): 940–7. doi:10.4088/JCP.v65n0710. PMID 15291683.
- Reeves EP, Williamson M, O'Neill SJ, Greally P, McElvaney NG (Jun 2011). "Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis". American Journal of Respiratory and Critical Care Medicine. 183 (11): 1517–23. doi:10.1164/rccm.201101-0072oc. PMID 21330456.
- Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J, Rot A, Schall T, Tsang M, Thorpe R, Van Damme J, Wadhwa M, Yoshie O, Zlotnik A, Zoon K (2002). "Chemokine/chemokine receptor nomenclature". J. Interferon Cytokine Res. 22 (10): 1067–8. doi:10.1089/107999002760624305. PMID 12433287.
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J (2009). "MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8". Aging. 1 (4): 402–11. doi:10.18632/aging.100042. PMC 2818025. PMID 20148189.
- Rottner M, Freyssinet JM, Martínez MC (2009). "Mechanisms of the noxious inflammatory cycle in cystic fibrosis". Respir. Res. 10 (1): 23. doi:10.1186/1465-9921-10-23. PMC 2660284. PMID 19284656.
- Ang Z, Abdi Gunawan Koen R, Er JZ, Lee LT, Tam Kit Chung J, Guo H, Ding JL (2019). "Novel AU-rich proximal UTR sequences (APS) enhance CXCL8 synthesis upon the induction of rpS6 phosphorylation". PLoS Genet. 15 (4): e1008077. doi:10.1371/journal.pgen.1008077. PMC 6476525. PMID 30969964.
Further reading
- Baggiolini M, Clark-Lewis I (1992). "Interleukin-8, a chemotactic and inflammatory cytokine". FEBS Lett. 307 (1): 97–101. doi:10.1016/0014-5793(92)80909-Z. PMID 1639201.
- Wahl SM, Greenwell-Wild T, Hale-Donze H, Moutsopoulos N, Orenstein JM (2000). "Permissive factors for HIV-1 infection of macrophages". J. Leukoc. Biol. 68 (3): 303–10. PMID 10985244.
- Starckx S, Van den Steen PE, Wuyts A, Van Damme J, Opdenakker G (2002). "Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization". Leuk. Lymphoma. 43 (2): 233–41. doi:10.1080/10428190290005982. PMID 11999552.
- Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP (2003). "Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion". Eur. Cytokine Netw. 13 (2): 161–72. PMID 12101072.
- Struyf S, Proost P, Van Damme J (2003). Regulation of the immune response by the interaction of chemokines and proteases. Adv. Immunol. Advances in Immunology. 81. pp. 1–44. doi:10.1016/S0065-2776(03)81001-5. ISBN 978-0-12-022481-4. PMID 14711052.
- Chakravorty M, Ghosh A, Choudhury A, Santra A, Hembrum J, Roychoudhury S (2004). "Ethnic differences in allele distribution for the IL8 and IL1B genes in populations from eastern India". Hum. Biol. 76 (1): 153–9. doi:10.1353/hub.2004.0016. PMID 15222686.
- Yuan A, Chen JJ, Yao PL, Yang PC (2005). "The role of interleukin-8 in cancer cells and microenvironment interaction". Front. Biosci. 10 (1–3): 853–65. doi:10.2741/1579. PMID 15569594.
- Copeland KF (2005). "Modulation of HIV-1 transcription by cytokines and chemokines". Mini Rev Med Chem. 5 (12): 1093–101. doi:10.2174/138955705774933383. PMID 16375755.