Interleukin 16
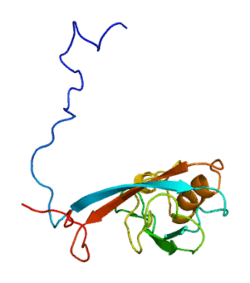
Pro-interleukin-16 is a protein that in humans is encoded by the IL16 gene.[5][6] This gene was discovered in 1982 at Boston University by Dr. David Center and Dr. William Cruikshank.[7]
Function
The protein encoded by this gene is a pleiotropic cytokine that functions as a chemoattractant, a modulator of T cell activation, and an inhibitor of HIV replication. The signaling process of this cytokine is mediated by CD4. The product of this gene undergoes proteolytic processing, which is found to yield two functional proteins. The cytokine function is exclusively attributed to the secreted C-terminal peptide, while the N-terminal product may play a role in cell cycle control. Caspase 3 is reported to be involved in the proteolytic processing of this protein. Two alternatively spliced transcript variants encoding distinct isoforms have been reported.[6]
Interleukin 16 (IL-16) is a cytokine that is released by a variety of cells (including lymphocytes and some epithelial cells) that has been characterized as a chemoattractant for certain immune cells expressing the cell surface molecule CD4.
IL-16 was originally described as a factor that could attract activated T cells in humans, it was previously called lymphocyte chemoattractant factor (LCF).[7] Since then, this interleukin has been shown to recruit and activate many other cells expressing the CD4 molecule, including monocytes, eosinophils, and dendritic cells.[8]
The structure of IL-16 was determined following its cloning in 1994.[9] This cytokine is produced as a precursor peptide (pro-IL-16) that requires processing by an enzyme called caspase-3 to become active. CD4 is the cell signaling receptor for mature IL-16.
Interactions
Interleukin 16 has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000172349 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000001741 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Baier M, Bannert N, Werner A, Lang K, Kurth R (Jun 1997). "Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor". Proc Natl Acad Sci U S A. 94 (10): 5273–7. doi:10.1073/pnas.94.10.5273. PMC 24668. PMID 9144227.
- "Entrez Gene: IL16 interleukin 16 (lymphocyte chemoattractant factor)".
- Cruikshank W, Center DM (1982). "Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF)". J. Immunol. 128 (6): 2569–74. PMID 7042841.
- Cruikshank WW, Kornfeld H, Center DM (2000). "Interleukin-16". J. Leukoc. Biol. 67 (6): 757–66. doi:10.1002/jlb.67.6.757. PMID 10857846.
- Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, Kornfeld H (1994). "Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression". Proc. Natl. Acad. Sci. U.S.A. 91 (11): 5109–13. doi:10.1073/pnas.91.11.5109. PMC 43941. PMID 7910967.
- Kurschner C, Yuzaki M (1999). "Neuronal interleukin-16 (NIL-16): a dual function PDZ domain protein". J. Neurosci. 19 (18): 7770–80. doi:10.1523/JNEUROSCI.19-18-07770.1999. PMC 6782450. PMID 10479680.
- Bannert N, Vollhardt K, Asomuddinov B, Haag M, König H, Norley S, Kurth R (2003). "PDZ Domain-mediated interaction of interleukin-16 precursor proteins with myosin phosphatase targeting subunits". J. Biol. Chem. 278 (43): 42190–9. doi:10.1074/jbc.M306669200. PMID 12923170.
Further reading
- Wilson KC, Center DM, Cruikshank WW (2005). "The effect of interleukin-16 and its precursor on T lymphocyte activation and growth". Growth Factors. 22 (2): 97–104. doi:10.1080/08977190410001704679. PMID 15253385.
- Copeland KF (2006). "Modulation of HIV-1 transcription by cytokines and chemokines". Mini Reviews in Medicinal Chemistry. 5 (12): 1093–101. doi:10.2174/138955705774933383. PMID 16375755.
- Rand TH, Cruikshank WW, Center DM, Weller PF (1991). "CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration". J. Exp. Med. 173 (6): 1521–8. doi:10.1084/jem.173.6.1521. PMC 2190841. PMID 1851800.
- Ryan TC, Cruikshank WW, Kornfeld H, et al. (1995). "The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration". J. Biol. Chem. 270 (29): 17081–6. doi:10.1074/jbc.270.29.17081. PMID 7615501.
- Cruikshank WW, Center DM, Nisar N, et al. (1994). "Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression". Proc. Natl. Acad. Sci. U.S.A. 91 (11): 5109–13. doi:10.1073/pnas.91.11.5109. PMC 43941. PMID 7910967.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Parada NA, Cruikshank WW, Danis HL, et al. (1996). "IL-16- and other CD4 ligand-induced migration is dependent upon protein kinase C". Cell. Immunol. 168 (1): 100–6. doi:10.1006/cimm.1996.0054. PMID 8599832.
- Bannert N, Baier M, Werner A, Kurth R (1996). "Interleukin-16 or not?". Nature. 381 (6577): 30. doi:10.1038/381030a0. PMID 8609984.
- Maciaszek JW, Parada NA, Cruikshank WW, et al. (1997). "IL-16 represses HIV-1 promoter activity". J. Immunol. 158 (1): 5–8. PMID 8977168.
- Laberge S, Ernst P, Ghaffar O, et al. (1997). "Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma". Am. J. Respir. Cell Mol. Biol. 17 (2): 193–202. CiteSeerX 10.1.1.319.2157. doi:10.1165/ajrcmb.17.2.2750. PMID 9271307.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Zhang Y, Center DM, Wu DM, et al. (1998). "Processing and activation of pro-interleukin-16 by caspase-3". J. Biol. Chem. 273 (2): 1144–9. doi:10.1074/jbc.273.2.1144. PMID 9422780.
- Mühlhahn P, Zweckstetter M, Georgescu J, et al. (1998). "Structure of interleukin 16 resembles a PDZ domain with an occluded peptide binding site". Nat. Struct. Biol. 5 (8): 682–6. doi:10.1038/1376. hdl:11858/00-001M-0000-002B-A4A1-7. PMID 9699630.
- Chupp GL, Wright EA, Wu D, et al. (1998). "Tissue and T cell distribution of precursor and mature IL-16". J. Immunol. 161 (6): 3114–9. PMID 9743378.
- Bannert N, Avots A, Baier M, et al. (1999). "GA-binding protein factors, in concert with the coactivator CREB binding protein/p300, control the induction of the interleukin 16 promoter in T lymphocytes". Proc. Natl. Acad. Sci. U.S.A. 96 (4): 1541–6. doi:10.1073/pnas.96.4.1541. PMC 15509. PMID 9990060.
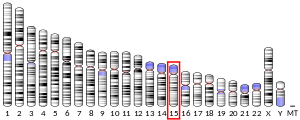
- Kim HS (1999). "Assignment of human interleukin 16 (IL16) to chromosome 15q26.3 by radiation hybrid mapping". Cytogenet. Cell Genet. 84 (1–2): 93. doi:10.1159/000015224. PMID 10343113.
- Liu Y, Cruikshank WW, O'Loughlin T, et al. (1999). "Identification of a CD4 domain required for interleukin-16 binding and lymphocyte activation". J. Biol. Chem. 274 (33): 23387–95. doi:10.1074/jbc.274.33.23387. PMID 10438516.
- Kaser A, Dunzendorfer S, Offner FA, et al. (1999). "A role for IL-16 in the cross-talk between dendritic cells and T cells". J. Immunol. 163 (6): 3232–8. PMID 10477592.
- Kurschner C, Yuzaki M (1999). "Neuronal interleukin-16 (NIL-16): a dual function PDZ domain protein". J. Neurosci. 19 (18): 7770–80. doi:10.1523/JNEUROSCI.19-18-07770.1999. PMID 10479680.
External links
- Overview of all the structural information available in the PDB for UniProt: Q14005 (Interleukin-16) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.