Blood donation
A blood donation occurs when a person voluntarily has blood drawn and used for transfusions and/or made into biopharmaceutical medications by a process called fractionation (separation of whole-blood components). Donation may be of whole blood, or of specific components directly (the latter called apheresis). Blood banks often participate in the collection process as well as the procedures that follow it.


Today in the developed world, most blood donors are unpaid volunteers who donate blood for a community supply. In some countries, established supplies are limited and donors usually give blood when family or friends need a transfusion (directed donation). Many donors donate as an act of charity, but in countries that allow paid donation some donors are paid, and in some cases there are incentives other than money such as paid time off from work. Donors can also have blood drawn for their own future use (autologous donation). Donating is relatively safe, but some donors have bruising where the needle is inserted or may feel faint.
Potential donors are evaluated for anything that might make their blood unsafe to use. The screening includes testing for diseases that can be transmitted by a blood transfusion, including HIV and viral hepatitis. The donor must also answer questions about medical history and take a short physical examination to make sure the donation is not hazardous to his or her health. How often a donor can donate varies from days to months based on what component they donate and the laws of the country where the donation takes place. For example, in the United States, donors must wait eight weeks (56 days) between whole blood donations but only seven days between plateletpheresis donations and twice per seven-day period in plasmapheresis.[1]
The amount of blood drawn and the methods vary. The collection can be done manually or with automated equipment that takes only specific components of the blood. Most of the components of blood used for transfusions have a short shelf life, and maintaining a constant supply is a persistent problem. This has led to some increased interest in autotransfusion, whereby a patient's blood is salvaged during surgery for continuous reinfusion—or alternatively, is "self-donated" prior to when it will be needed. (Generally, the notion of "donation" does not refer to giving to one's self, though in this context it has become somewhat acceptably idiomatic.)
Types of donation

Blood donations are divided into groups based on who will receive the collected blood.[2] An 'allogeneic' (also called 'homologous') donation is when a donor gives blood for storage at a blood bank for transfusion to an unknown recipient. A 'directed' donation is when a person, often a family member, donates blood for transfusion to a specific individual.[3] Directed donations are relatively rare when an established supply exists.[4] A 'replacement donor' donation is a hybrid of the two and is common in developing countries such as Ghana.[5] In this case, a friend or family member of the recipient donates blood to replace the stored blood used in a transfusion, ensuring a consistent supply. When a person has blood stored that will be transfused back to the donor at a later date, usually after surgery, that is called an 'autologous' donation.[6] Blood that is used to make medications can be made from allogeneic donations or from donations exclusively used for manufacturing.[7]
Blood is sometimes collected using similar methods for therapeutic phlebotomy, similar to the ancient practice of bloodletting, which is used to treat conditions such as hereditary hemochromatosis or polycythemia vera. This blood is sometimes treated as a blood donation, but may be immediately discarded if it cannot be used for transfusion or further manufacturing.
The actual process varies according to the laws of the country, and recommendations to donors vary according to the collecting organization.[8][9][10] The World Health Organization gives recommendations for blood donation policies,[11] but in developing countries many of these are not followed. For example, the recommended testing requires laboratory facilities, trained staff, and specialized reagents, all of which may not be available or too expensive in developing countries.[12]
An event where donors come to donate allogeneic blood is sometimes called a 'blood drive' or a 'blood donor session'. These can occur at a blood bank, but they are often set up at a location in the community such as a shopping center, workplace, school, or house of worship.[13]
Screening
Donors are typically required to give consent for the process and this requirement means minors cannot donate without permission from a parent or guardian.[14] In some countries, answers are associated with the donor's blood, but not name, to provide anonymity; in others, such as the United States, names are kept to create lists of ineligible donors.[15] If a potential donor does not meet these criteria, they are 'deferred'. This term is used because many donors who are ineligible may be allowed to donate later. Blood banks in the United States may be required to label the blood if it is from a therapeutic donor, so some do not accept donations from donors with any blood disease.[16] Others, such as the Australian Red Cross Blood Service, accept blood from donors with hemochromatosis. It is a genetic disorder that does not affect the safety of the blood.[17]
The donor's race or ethnic background is sometimes important since certain blood types, especially rare ones, are more common in certain ethnic groups.[18] Historically, in the United States donors were segregated or excluded on race, religion, or ethnicity, but this is no longer a standard practice.[19][20]
Recipient safety
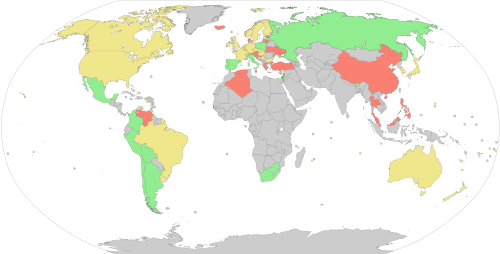
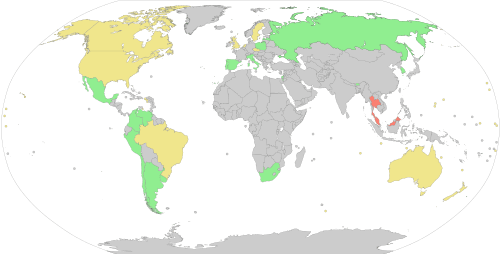
Donors are screened for health risks that could make the donation unsafe for the recipient. Some of these restrictions are controversial, such as restricting donations from men who have sex with men (MSM) because of the risk of transmitting HIV.[21] In 2011, the UK (excluding Northern Ireland) reduced its blanket ban on MSM donors to a narrower restriction which only prevents MSM from donating blood if they have had sex with other men within the past year.[22] A similar change was made in the U.S. in late 2015 by the FDA.[23] In 2017, the UK further reduced its restriction to three months.[24] Autologous donors are not always screened for recipient safety problems since the donor is the only person who will receive the blood.[25] Since the donated blood may be given to pregnant women or women of child-bearing age, donors taking teratogenic (birth defect causing) medications are deferred. These medications include acitretin, etretinate, isotretinoin, finasteride and dutasteride.[26]
Donors are examined for signs and symptoms of diseases that can be transmitted in a blood transfusion, such as HIV, malaria, and viral hepatitis. Screening may include questions about risk factors for various diseases, such as travel to countries at risk for malaria or variant Creutzfeldt–Jakob disease (vCJD). These questions vary from country to country. For example, while blood centers in Québec, Poland, and many other places defer donors who lived in the United Kingdom for risk of vCJD,[27][28] donors in the United Kingdom are only restricted for vCJD risk if they have had a blood transfusion in the United Kingdom.[29]
Donor safety
The donor is also examined and asked specific questions about their medical history to make sure that donating blood is not hazardous to their health. The donor's hematocrit or hemoglobin level is tested to make sure that the loss of blood will not make them anemic, and this check is the most common reason that a donor is ineligible.[30] Pulse, blood pressure, and body temperature are also evaluated. Elderly donors are sometimes also deferred on age alone because of health concerns.[31] The safety of donating blood during pregnancy has not been studied thoroughly, and pregnant women are usually deferred until six weeks after the pregnancy.[32]
Blood testing
The donor's blood type must be determined if the blood will be used for transfusions. The collecting agency usually identifies whether the blood is type A, B, AB, or O and the donor's Rh (D) type and will screen for antibodies to less common antigens. More testing, including a crossmatch, is usually done before a transfusion. Type O negative is often cited as the "universal donor"[33] but this only refers to red cell and whole blood transfusions. For plasma and platelet transfusions the system is reversed: AB positive is the universal platelet donor type while both AB positive and AB negative are universal plasma donor types.[34][35]
Most blood is tested for diseases, including some STDs.[36] The tests used are high-sensitivity screening tests and no actual diagnosis is made. Some of the test results are later found to be false positives using more specific testing.[37] False negatives are rare, but donors are discouraged from using blood donation for the purpose of anonymous STD screening because a false negative could mean a contaminated unit. The blood is usually discarded if these tests are positive, but there are some exceptions, such as autologous donations. The donor is generally notified of the test result.[38]
Donated blood is tested by many methods, but the core tests recommended by the World Health Organization are these four:
- Hepatitis B Surface Antigen
- Antibody to Hepatitis C
- Antibody to HIV, usually subtypes 1 and 2
- Serologic test for Syphilis
The WHO reported in 2006 that 56 out of 124 countries surveyed did not use these basic tests on all blood donations.[12]
A variety of other tests for transfusion transmitted infections are often used based on local requirements. Additional testing is expensive, and in some cases the tests are not implemented because of the cost.[39] These additional tests include other infectious diseases such as West Nile fever,[40] and babesiosis.[41] Sometimes multiple tests are used for a single disease to cover the limitations of each test. For example, the HIV antibody test will not detect a recently infected donor, so some blood banks use a p24 antigen or HIV nucleic acid test in addition to the basic antibody test to detect infected donors during that period. Cytomegalovirus is a special case in donor testing in that many donors will test positive for it.[42] The virus is not a hazard to a healthy recipient, but it can harm infants[43] and other recipients with weak immune systems.[42]
Obtaining the blood
There are two main methods of obtaining blood from a donor. The most frequent is to simply take the blood from a vein as whole blood. This blood is typically separated into parts, usually red blood cells and plasma, since most recipients need only a specific component for transfusions. A typical donation is 450 millilitres (or approximately one U.S. pint)[44] of whole blood, though 500 millilitre donations are also common. Historically, blood donors in India would donate only 250 or 350 millilitre and donors in the People's Republic of China would donate only 200 millilitres, though larger 300 and 400 millilitre donations have become more common.[45]
The other method is to draw blood from the donor, separate it using a centrifuge or a filter, store the desired part, and return the rest to the donor. This process is called apheresis, and it is often done with a machine specifically designed for this purpose. This process is especially common for plasma and platelets.
For direct transfusions a vein can be used but the blood may be taken from an artery instead.[46] In this case, the blood is not stored, but is pumped directly from the donor into the recipient. This was an early method for blood transfusion and is rarely used in modern practice.[47] It was phased out during World War II because of problems with logistics, and doctors returning from treating wounded soldiers set up banks for stored blood when they returned to civilian life.[48]
Site preparation and drawing blood
The blood is drawn from a large arm vein close to the skin, usually the median cubital vein on the inside of the elbow. The skin over the blood vessel is cleaned with an antiseptic such as iodine or chlorhexidine[49] to prevent skin bacteria from contaminating the collected blood[49] and also to prevent infections where the needle pierced the donor's skin.[50]
A large[51] needle (16 to 17 gauge) is used to minimize shearing forces that may physically damage red blood cells as they flow through the needle.[52] A tourniquet is sometimes wrapped around the upper arm to increase the pressure of the blood in the arm veins and speed up the process. The donor may also be prompted to hold an object and squeeze it repeatedly to increase the blood flow through the vein.
Whole blood
The most common method is collecting the blood from the donor's vein into a container. The amount of blood drawn varies from 200 millilitres to 550 millilitres depending on the country, but 450–500 millilitres is typical.[42] The blood is usually stored in a flexible plastic bag that also contains sodium citrate, phosphate, dextrose, and adenine. This combination keeps the blood from clotting and preserves it during storage up to 42 days.[53][54][55] Other chemicals are sometimes added during processing.
The plasma from whole blood can be used to make plasma for transfusions or it can also be processed into other medications using a process called fractionation. This was a development of the dried plasma used to treat the wounded during World War II and variants on the process are still used to make a variety of other medications.[56][57]
Apheresis
Apheresis is a blood donation method where the blood is passed through an apparatus that separates out one particular constituent and returns the remainder to the donor. Usually the component returned is the red blood cells, the portion of the blood that takes the longest to replace. Using this method an individual can donate plasma or platelets much more frequently than they can safely donate whole blood.[58] These can be combined, with a donor giving both plasma and platelets in the same donation.
Platelets can also be separated from whole blood, but they must be pooled from multiple donations. From three to ten units of whole blood are required for a therapeutic dose.[59] Plateletpheresis provides at least one full dose from each donation.
During a platelet donation, the blood is drawn from the patient and the platelets are separated from the other blood components. The remainder of the blood, red blood cells, plasma, and white blood cells are returned to the patient. This process is completed several times for a period of two hours to collect a single donation.[60]
Plasmapheresis is frequently used to collect source plasma that is used for manufacturing into medications much like the plasma from whole blood. Plasma collected at the same time as plateletpheresis is sometimes called concurrent plasma.
Apheresis is also used to collect more red blood cells than usual in a single donation (commonly known as "double reds") and to collect white blood cells for transfusion.[61][62]
Recovery and time between donations
Donors are usually kept at the donation site for 10–15 minutes after donating since most adverse reactions take place during or immediately after the donation.[63] Blood centers typically provide light refreshments, such as orange juice and cookies, or a lunch allowance to help the donor recover.[64] The needle site is covered with a bandage and the donor is directed to keep the bandage on for several hours.[44] In hot climates, donors are advised to avoid dehydration (strenuous exercise and games, alcohol) until a few hours after donation.
Donated plasma is replaced after 2–3 days.[65] Red blood cells are replaced by bone marrow into the circulatory system at a slower rate, on average 36 days in healthy adult males. In one study, the range was 20 to 59 days for recovery.[66] These replacement rates are the basis of how frequently a donor can donate blood.
Plasmapheresis and plateletpheresis donors can donate much more frequently because they do not lose significant amounts of red cells. The exact rate of how often a donor can donate differs from country to country. For example, plasmapheresis donors in the United States are allowed to donate large volumes twice a week and could nominally donate 83 litres (about 22 gallons) in a year, whereas the same donor in Japan may only donate every other week and could only donate about 16 litres (about 4 gallons) in a year.[67]
Iron supplementation decreases the rates of donor deferral due to low hemoglobin, both at the first donation visit and at subsequent donations. Iron-supplemented donors have higher hemoglobin and iron stores. On the other hand, iron supplementation frequently causes diarrhea, constipation and epigastric abdominal discomfort. The long-term effects of iron supplementation without measurement of iron stores are unknown.[68]
Complications
Donors are screened for health problems that would put them at risk for serious complications from donating. First-time donors, teenagers, and women are at a higher risk of a reaction.[69][70] One study showed that 2% of donors had an adverse reaction to donation.[71] Most of these reactions are minor. A study of 194,000 donations found only one donor with long-term complications.[72] In the United States, a blood bank is required to report any death that might possibly be linked to a blood donation. An analysis of all reports from October 2008 to September 2009 evaluated six events and found that five of the deaths were clearly unrelated to donation, and in the remaining case they found no evidence that the donation was the cause of death.[73]
Hypovolemic reactions can occur because of a rapid change in blood pressure. Fainting is generally the worst problem encountered.[74]
The process has similar risks to other forms of phlebotomy. Bruising of the arm from the needle insertion is the most common concern. One study found that less than 1% of donors had this problem.[75] A number of less common complications of blood donation are known to occur. These include arterial puncture, delayed bleeding, nerve irritation, nerve injury, tendon injury, thrombophlebitis, and allergic reactions.[76]
Donors sometimes have adverse reactions to the sodium citrate used in apheresis collection procedures to keep the blood from clotting. Since the anticoagulant is returned to the donor along with blood components that are not being collected, it can bind the calcium in the donor's blood and cause hypocalcemia.[77] These reactions tend to cause tingling in the lips, but may cause convulsions, seizure, hypertension, or more serious problems.[78] Donors are sometimes given calcium supplements during the donation to prevent these side effects.[79]
In apheresis procedures, the red blood cells are returned. If this is done manually and the donor receives the blood from a different donor, a transfusion reaction can take place. Manual apheresis is extremely rare in the developed world because of this risk and automated procedures are as safe as whole blood donations.[80]
The final risk to blood donors is from equipment that has not been properly sterilized.[81] In most cases, the equipment that comes in direct contact with blood is discarded after use.[82] Re-used equipment was a significant problem in China in the 1990s, and up to 250,000 blood plasma donors may have been exposed to HIV from shared equipment.[83][84][85]
Storage, supply and demand
_(9759061442).jpg)
Storage and blood shelf life
The collected blood is usually stored in a blood bank as separate components, and some of these have short shelf lives. There are no storage methods to keep platelets for extended periods of time, though some were being studied as of 2008.[86] The longest shelf life used for platelets is seven days.[87]
Red blood cells (RBC), the most frequently used component, have a shelf life of 35–42 days at refrigerated temperatures.[88][89] For (relatively rare) long-term storage applications, this can be extended by freezing the blood with a mixture of glycerol, but this process is expensive and requires an extremely cold freezer for storage.[42] Plasma can be stored frozen for an extended period of time and is typically given an expiration date of one year and maintaining a supply is less of a problem.[90]
Demand for blood
The limited storage time means that it is difficult to have a stockpile of blood to prepare for a disaster. The subject was discussed at length after the September 11 attacks in the United States, and the consensus was that collecting during a disaster was impractical and that efforts should be focused on maintaining an adequate supply at all times.[91] Blood centers in the U.S. often have difficulty maintaining even a three-day supply for routine transfusion demands.[92]
Donation levels
The World Health Organization (WHO) recognizes World Blood Donor Day on 14 June each year to promote blood donation. This is the birthday of Karl Landsteiner, the scientist who discovered the ABO blood group system.[93] The theme of the 2012 World Blood Donor Day campaign, "Every blood donor is a hero" focuses on the idea that everyone can become a hero by giving blood. Based on data reported by 180 countries between 2011 and 2013, the WHO estimated that approximately 112.5 million units of blood were being collected annually.[94]
In the United States it is estimated that 111 million citizens are eligible blood donors,[95] or 37% of the population.[96] However less than 10% of the 37% eligible blood donors donate annually.[96] In the UK the NHS reports blood donation levels at "only 4%"[97] while in Canada the rate is 3.5%.[98]
Donor health benefits
In patients prone to iron overload, blood donation prevents the accumulation of toxic quantities.[99] Donating blood may reduce the risk of heart disease for men, but the link has not been firmly established and may be from selection bias because donors are screened for health problems.[100][101]
Research published in 2012 demonstrated that repeated blood donation is effective in reducing blood pressure, blood glucose, HbA1c, low-density lipoprotein/high-density lipoprotein ratio, and heart rate in patients with metabolic syndrome.[102]
Donor compensation


The World Health Organization set a goal in 1997 for all blood donations to come from unpaid volunteer donors, but as of 2006, only 49 of 124 countries surveyed had established this as a standard.[12] Some countries, such as Tanzania, have made great strides in moving towards this standard, with 20 percent of donors in 2005 being unpaid volunteers and 80 percent in 2007, but 68 of 124 countries surveyed by WHO had made little or no progress.[5] Most plasmapheresis donors in the United States are still paid for their donations.[103] Donors are now paid between $25 and $50 per donation.[104] In some countries, for example Australia, Brazil and Great Britain, it is illegal to receive any compensation, monetary or otherwise, for the donation of blood or other human tissues.[105][106]
Regular donors are often given some sort of non-monetary recognition. Time off from work is a common benefit.[107] For example, in Italy, blood donors receive the donation day as a paid holiday from work.[108] Blood centers will also sometimes add incentives such as assurances that donors would have priority during shortages, free T-shirts, first aid kits, windshield scrapers, pens, and similar trinkets. There are also incentives for the people who recruit potential donors, such as prize drawings for donors and rewards for organizers of successful drives.[109] Recognition of dedicated donors is common. For example, the Singapore Red Cross Society presents awards for voluntary donors who have made a certain number of donations under the Blood Donor Recruitment Programme starting with a "bronze award" for 25 donations.[110] The government of Malaysia also offers free outpatient and hospitalization benefits for blood donors, for example, 4 months of free outpatient treatment and hospitalization benefits after every donation.[111] In Poland, after donating a specific amount of blood (18 litres for men and 15 for women), a person is gifted with the title of "Distinguished Honorary Blood Donor" as well as a medal. In addition, a popular privilege in larger Polish cities is the right to free use of public transport, but the conditions for obtaining a privilege may vary depending on the city.
Most allogeneic blood donors donate as an act of charity and do not expect to receive any direct benefit from the donation.[112] The sociologist Richard Titmuss, in his 1970 book The Gift Relationship: From Human Blood to Social Policy, compared the merits of the commercial and non-commercial blood donation systems of the US and the UK, coming down in favor of the latter. The book became a bestseller in the US, resulting in legislation to regulate the private market in blood.[113] The book is still referenced in modern debates about turning blood into a commodity.[114] The book was republished in 1997 and the same ideas and principles are applied to analogous donation programs, such as organ donation and sperm donation.[115]
See also
- Blood substitutes
- History of blood donation
- James Harrison (blood donor)
- List of blood donation agencies
- Men who have sex with men blood donor controversy
- Xenotransfusion
References
- "FAQs About Donating Blood". American Red Cross Biomedical Services. Archived from the original on 2009-10-20. Retrieved 2009-10-26.
- M. E. Brecher, Editor (2005), AABB Technical Manual, 15th edition, Bethesda, MD: AABB, ISBN 1-56395-196-7, pp. 98–103
- "Directed Donation". Mayo Clinic. Archived from the original on 2008-05-24. Retrieved 2008-06-25.
- Wales PW, Lau W, Kim PC (May 2001). "Directed blood donation in pediatric general surgery: Is it worth it?". J. Pediatr. Surg. 36 (5): 722–25. doi:10.1053/jpsu.2001.22945. PMID 11329574.
- T. Brown "Strengthening Blood Systems In Africa: Progress Under PEPFAR and Remaining Challenges" AABB News. April 1998: p. 30
- "Autologous (self-donated) Blood as an Alternative to Allogeneic (donor-donated) Blood Transfusion". AABB. Archived from the original on 2008-06-12. Retrieved 2008-06-25.
- "Recovered Plasma". AABB. Archived from the original on 2008-06-12. Retrieved 2008-06-25.
- "Giving Blood -> What to Expect". Australian Red Cross Blood Service. Archived from the original on 2007-08-29. Retrieved 2007-10-06.
- "The Donation Experience". Canadian Blood Services. Archived from the original on 2007-02-03. Retrieved 2006-12-17.
- "Tips for a Good Donation Experience". American Red Cross. Archived from the original on 2006-12-14. Retrieved 2006-12-17.
- "WHO Blood Safety and Donation". World Health Organization. Archived from the original on 2008-06-29. Retrieved 2008-06-01.
- "World Blood Donor Day 2006". World Health Organization. Archived from the original on 2008-06-17. Retrieved 2008-06-26.
- "Sponsoring a Blood Drive". American Red Cross. Archived from the original on 2008-05-26. Retrieved 2008-06-25.
- "Parental consent form" (PDF). Australian Red Cross Blood Service. Retrieved 2008-06-01.
- "FDA regulations on donor deferral". US Food and Drug Administration. Archived from the original on 2008-05-14. Retrieved 2008-06-01.
- "Variances for Blood Collection from Individuals with Hereditary Hemochromatosis". US Food and Drug Administration. Archived from the original on 2007-07-08. Retrieved 2007-07-18.
- "Hereditary Hemochromatosis: Perspectives of Public Health, Medical Genetics, and Primary Care". CDC Office of Public Health Genomics. Archived from the original on 2008-03-08. Retrieved 2008-06-03.
- Severo, Richard (1990-01-13). "Donors' Races to Be Sought To Identify Rare Blood Types". The New York Times. Archived from the original on 2009-03-03. Retrieved 2008-06-01.
- "Red Gold, Innovators and Pioneers". Public Broadcasting Service (United States). Archived from the original on 2008-05-17. Retrieved 2008-06-01.
- "How Science Students Helped End Segregated Blood Banks". National Center For Science Education (United States). 2015-08-05. Archived from the original on 2017-11-07. Retrieved 2017-11-05.
- "Drug Agency Reaffirms Ban on Gay Men Giving Blood". The New York Times. 2007-05-24. Archived from the original on 2013-05-24. Retrieved 2009-03-26.
- "HIV charities welcome the lifting of lifetime ban on gay men donating blood". National Aids Trust. 2011-09-08. Archived from the original on 2014-02-02. Retrieved 2014-01-31.
- "Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products - Questions and Answers". US Food and Drug Administration. Archived from the original on 2016-03-30. Retrieved 2016-03-30.
- "The rules on blood donation in England change on 28th November". NHS Blood and Transplant. 2017-11-28. Archived from the original on 2019-10-15. Retrieved 2019-10-21.
- Heim MU, Mempel W (1991). "[The need for thorough infection screening in donors of autologous blood]". Beitr Infusionsther (in German). 28: 313–16. PMID 1725645.
- "Avodart consumer information". US Food and Drug Administration. Archived from the original on May 9, 2009. Retrieved 2008-06-01.
- "Donor Qualification criteria". Héma-Québec, Canada. Archived from the original on 2006-11-08. Retrieved 2006-12-17.
- "Permanent exclusion criteria / Dyskwalifikacja stała" (in Polish). RCKiK Warszawa. Archived from the original on August 30, 2009. Retrieved 2010-03-03.
- "Guidelines for UK Blood Services". UK Blood and Tissue Services. Archived from the original on 2008-05-15. Retrieved 2008-06-01.
- Gómez-Simón A; Navarro-Núñez L; Pérez-Ceballos E; et al. (Jun 2007). "Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings". Transfus. Apher. Sci. 36 (3): 235–42. doi:10.1016/j.transci.2007.01.010. PMID 17556020.
- Goldman M, Fournier E, Cameron-Choi K, Steed T (May 2007). "Effect of changing the age criteria for blood donors". Vox Sang. 92 (4): 368–72. doi:10.1111/j.1423-0410.2007.00897.x. PMID 17456161.
- "Donating – Frequently Asked Questions". Blood Bank of Alaska. Archived from the original on 2008-06-06. Retrieved 2008-06-01.
- "Blood Type Test". WebMD.com. Archived from the original on 2008-05-29. Retrieved 2008-06-01.
- "Plasma fact sheet" (PDF). American Red Cross. Archived from the original (PDF) on 2009-03-26.
- "Right Type, Right Time".
- Bhattacharya P; Chandra PK; Datta S; et al. (Jul 2007). "Significant increase in HBV, HCV, HIV and syphilis infections among blood donors in West Bengal, Eastern India 2004–2005: exploratory screening reveals high frequency of occult HBV infection". World J. Gastroenterol. 13 (27): 3730–33. doi:10.3748/wjg.v13.i27.3730. PMC 4250646. PMID 17659734. Archived from the original on 2008-12-29.
- "Testing of Donor Blood for infectious disease". AABB. Archived from the original on 2008-05-09. Retrieved 2008-06-25.
- R. Miller; P.E. Hewitt; R. Warwick; M.C. Moore; B. Vincent (1998). "Review of counselling in a transfusion service: the London (UK) experience". Vox Sang. 74 (3): 133–39. doi:10.1046/j.1423-0410.1998.7430133.x. PMID 9595639.
- "Advisory Committee on MSBTO, 28 June 2005" (PDF). Archived from the original (PDF) on 28 May 2008. Retrieved 2008-06-01.
- "Precautionary West Nile virus blood sample testing". Héma-Québec, Canada. Archived from the original on 2007-09-13. Retrieved 2006-12-17.
- "FDA approves first tests to screen for tickborne parasite in whole blood and plasma to protect the U.S. blood supply" (Press release). FDA. 6 March 2018. Archived from the original on 7 March 2018. Retrieved 6 March 2018.
While babesiosis is both preventable and treatable, until today, there was no way to screen for infections amongst blood donors
- "Circular of Information for use of Blood and Blood Products" (PDF). AABB, ARC, America's Blood Centers. Archived from the original (PDF) on 2009-10-07.
- "Red blood cell transfusions in newborn infants: Revised guidelines". Canadian Paediatric Society (CPS). Archived from the original on 2007-02-03. Retrieved 2007-02-02.
- "Blood donation: What to expect". Mayo Clinic. Archived from the original on 2008-12-04. Retrieved 2008-12-03.
- Jingxing Wang; Nan Guo (corresponding); et al. (December 2010). "Who donates blood at five ethnically and geographically diverse blood centers in China in 2008". Transfusion. 50 (12): 2686–94. doi:10.1111/j.1537-2995.2010.02722.x. PMID 20553435.
- Sagi E, Eyal F, Armon Y, Arad I, Robinson M (Nov 1981). "Exchange transfusion in newborns via a peripheral artery and vein". Eur. J. Pediatr. 137 (3): 283–84. doi:10.1007/BF00443258. PMID 7318840.
- "Blood on the Hoof". Public Broadcasting Service. Archived from the original on 2008-06-04. Retrieved 2008-06-25.
- "ISBT Quarterly Newsletter, June 2006, "A History of Fresh Blood", p. 15" (PDF). International Society of Blood Transfusion (ISBT/SITS). Archived from the original (PDF) on 2008-08-03. Retrieved 2008-07-31.
- Lee CK, Ho PL, Chan NK, Mak A, Hong J, Lin CK (Oct 2002). "Impact of donor arm skin disinfection on the bacterial contamination rate of platelet concentrates". Vox Sang. 83 (3): 204–08. doi:10.1046/j.1423-0410.2002.00219.x. PMID 12366760.
- M. L. Turgeon (2004). Clinical Hematology: Theory and Procedures (fourth ed.). Lippincott Williams & Wilkins. p. 30. ISBN 978-0-7817-5007-3. Retrieved 2008-06-21.
- One major manufacturer of collection sets uses a 16 gauge (1.651 mm) size "Blood banking laboratory supplies" (PDF). Genesis BPS. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- "What is Hemolysis?" (PDF). Becton-Dickinson. Archived (PDF) from the original on 2008-06-25. Retrieved 2008-06-01.
- Akerblom O, Kreuger A (1975). "Studies on citrate-phosphate-dextrose (CPD) blood supplemented with adenine". Vox Sang. 29 (2): 90–100. doi:10.1111/j.1423-0410.1975.tb00484.x. PMID 238338.
- Sugita, Yoshiki; Simon, Ernest R. (1965). "The Mechanism of Action of Adenine in Red Cell Preservation*". Journal of Clinical Investigation. 44 (4): 629–642. doi:10.1172/JCI105176. ISSN 0021-9738. PMC 292538. PMID 14278179.
- Simon, Ernest R.; Chapman, Robert G.; Finch, Clement A. (1962). "Adenine in red cell preservation". Journal of Clinical Investigation. 41 (2): 351–359. doi:10.1172/JCI104489. ISSN 0021-9738. PMC 289233. PMID 14039291.
- "Plasma Equipment and Packaging, and Transfusion Equipment". Office of Medical History (OTSG). Archived from the original on 2017-06-09. Retrieved 2008-06-19.
- "Medicines derived from human plasma". Sanquin Blood Supply Foundation. Archived from the original on 2009-01-01. Retrieved 2008-06-01.
- Component Donation Archived 2009-10-11 at the Wayback Machine UK National Blood Service. Retrieved 2009-10-26
- "Indications for Platelet Transfusion Therapy". Southeastern Community Blood Center. Archived from the original on 2008-05-12. Retrieved 2008-06-10.
- "Platelet Donation". The American National Red Cross. Archived from the original on 2019-03-29. Retrieved 2019-05-16.
- "Double Up to Save Lives". United Blood Services. Archived from the original on 2006-12-30. Retrieved 2007-02-23.
- "Double the Difference". American Red Cross (Greater Chesapeake and Potomac). Archived from the original on 2007-05-13. Retrieved 2007-02-23.
- Eder AF, Hillyer CD, Dy BA, Notari EP, Benjamin RJ (May 2008). "Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds". JAMA. 299 (19): 2279–86. doi:10.1001/jama.299.19.2279. PMID 18492969.
- "Report on the promotion by Member States of voluntary unpaid blood donation" (PDF). Commission of the European Communities. Archived from the original (PDF) on 2008-08-03. Retrieved 2008-06-26.
- "Donating Apheresis and Plasma". Community Blood Center. Archived from the original on 2008-07-04. Retrieved 2008-06-11.
- Pottgiesser T, Specker W, Umhau M, Dickhuth HH, Roecker K, Schumacher YO (2008). "Recovery of hemoglobin mass after blood donation". Transfusion. 48 (7): 1390–1397. doi:10.1111/j.1537-2995.2008.01719.x. PMID 18466177.
- "Blood Products Advisory Committee, 12 December 2003". Archived from the original on 8 November 2008. Retrieved 2008-06-01.
- Smith GA, Fisher SA, Doree C, Di Angelantonio E, Roberts DJ (2014). "Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors". Cochrane Database Syst Rev. 7 (7): CD009532. doi:10.1002/14651858.CD009532.pub2. PMID 24990381.
- A.F. Eder; C.D. Hillyer; B.A. Dy; E.P. Notari; R.J. Benjamin (May 2008). "Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds". JAMA. 299 (19): 2279–86. doi:10.1001/jama.299.19.2279. PMID 18492969.
- Yuan S, Gornbein J, Smeltzer B, Ziman AF, Lu Q, Goldfinger D (Jun 2008). "Risk factors for acute, moderate to severe donor reactions associated with multicomponent apheresis collections". Transfusion. 48 (6): 1213–19. doi:10.1111/j.1537-2995.2008.01674.x. PMID 18346014.
- "Adverse Effect of Blood Donation, Siriraj Experience" (PDF). American Red Cross. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- B. Newman; S. Graves (2001). "A study of 178 consecutive vasovagal syncopal reactions from the perspective of safety". Transfusion. 41 (12): 1475–79. doi:10.1046/j.1537-2995.2001.41121475.x. PMID 11778059.
- "Fatalities Reported to FDA". US Food and Drug Administration. Archived from the original on 2011-01-02. Retrieved 2010-12-20.
- Wiltbank TB, Giordano GF, Kamel H, Tomasulo P, Custer B (May 2008). "Faint and prefaint reactions in whole-blood donors: an analysis of predonation measurements and their predictive value". Transfusion. 48 (9): 1799–808. doi:10.1111/j.1537-2995.2008.01745.x. PMID 18482188.
- Ranasinghe E, Harrison JF (Jun 2000). "Bruising following blood donation, its management and the response and subsequent return rates of affected donors". Transfus Med. 10 (2): 113–16. doi:10.1046/j.1365-3148.2000.00240.x. PMID 10849380.
- Working Group on Complications Related to Blood Donation JF (2008). "Standard for Surveillance of Complications Related to Blood D Donation" (PDF). European Haemovigilance Network: 11. Archived from the original (PDF) on 2010-02-15.
- Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF (Sep 2001). "Comprehensive analysis of citrate effects during plateletpheresis in normal donors". Transfusion. 41 (9): 1165–71. doi:10.1046/j.1537-2995.2001.41091165.x. PMID 11552076.
- Sorensen, BS; Johnsen, SP; Jorgensen, J. (Feb 2008). "Complications related to blood donation: A population-based study". Vox Sanguinis. 94 (2): 132–37. doi:10.1111/j.1423-0410.2007.01000.x. PMID 18028259.
- "Jerome H. Holland Laboratory for the Biomedical Sciences Volunteer Research Blood Program (RBP)". American Red Cross. Archived from the original on 2008-03-15. Retrieved 2008-06-01.
- Wiltbank TB, Giordano GF; Giordano, Gerald F. (Jun 2007). "The safety profile of automated collections: an analysis of more than 1 million collections". Transfusion. 47 (6): 1002–05. doi:10.1111/j.1537-2995.2007.01224.x. PMID 17524089.
- Global AIDS Crisis: A Reference Handbook, Richard G. Marlink, Alison G. Kotin, p. 16 , ABC-CLIO
- "Blood Donor Information Leaflet". Irish Blood Transfusion Service. Archived from the original on 2007-11-19. Retrieved 2008-06-01.
- "Keeping China's blood supply free of HIV". US Embassy, Beijing. Archived from the original on 2008-09-07. Retrieved 2008-06-01.
- Cohen J (Jun 2004). "HIV/AIDS in China. An unsafe practice turned blood donors into victims". Science. 304 (5676): 1438–39. doi:10.1126/science.304.5676.1438. PMID 15178781.
- Busby, Mattha (26 September 2019). "Contaminated blood whistleblower dies in US". The Guardian. Retrieved 26 September 2019.
- Beard, M.; Garwood, M.; Cookson, P.; Bashir, S.; Hancock, V.; Pergande, C.; Smith, K.; Turner, C.; Wiltshire, M.; Thomas, S.; Cardigan, R. (2006). "In Vitro Evaluation of Buffy Coat Derived Platelet Concentrates in SSP+ Platelet Storage Medium". Transfusion Medicine. 16: 26. doi:10.1111/j.1365-3148.2006.00694_4.x.
- "Transfusion Handbook, summary information for Platelets". National Blood Transfusion Committee. Archived from the original on 2008-08-04. Retrieved 2008-06-02.
- Lockwood WB, Hudgens RW, Szymanski IO, Teno RA, Gray AD (Nov 2003). "Effects of rejuvenation and frozen storage on 42-day-old AS-3 RBCs". Transfusion. 43 (11): 1527–32. doi:10.1046/j.1537-2995.2003.00551.x. PMID 14617310.
- "Transfusion handbook, Summary information for Red Blood Cells". National Blood Transfusion Committee. Archived from the original on 2008-08-04. Retrieved 2008-06-02.
- "Transfusion of Fresh Frozen Plasma, products, indications" (PDF). Agence française de sécurité sanitaire des produits de santé. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-02.
- "Maintaining an Adequate Blood Supply Is Key to Emergency Preparedness" (PDF). Government Accountability Office. Archived (PDF) from the original on 2013-12-24. Retrieved 2008-06-01.
- "Current status of America's Blood Centers blood supply". America's Blood Centers. Archived from the original on 2009-02-01.
- "World Blood Donor Day". World Health Organization. Archived from the original on 2008-09-15. Retrieved 2008-06-01.
- "Blood safety and availability". World Health Organization. Retrieved 2019-01-11.
- Riley W, Schwei M, McCullough J (July 2007). "The United States' potential blood donor pool: estimating the prevalence of donor-exclusion factors on the pool of potential donors" (PDF). Transfusion. 47 (7): 1180–88. doi:10.1111/j.1537-2995.2007.01252.x. PMID 17581152. Archived from the original (PDF) on 2012-09-27. Retrieved 2013-01-04.
- "Facts About Blood". America's Blood Centers. Archived from the original on 2013-11-08. Retrieved 2013-01-04.
- "Give Blood". NHS Blood & Transfustion. Archived from the original on 2011-12-30. Retrieved 2012-01-04.
Blood is something we all expect to be there for us when we need it, yet only 4% of us give blood.
- "Canadian Blood Services". Archived from the original on 2013-05-23.
- Fields AC, Grindon AJ (1999). "Hemochromatosis, iron, and blood donation: a short review". Immunohematology. 15 (3): 108–12. PMID 15373512.
- Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT (Mar 1997). "Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland". BMJ. 314 (7083): 793–94. doi:10.1136/bmj.314.7083.793. PMC 2126176. PMID 9080998.
- Atsma, F.; de Vegt, F. (Sep 2011). "The healthy donor effect: a matter of selection bias and confounding". Transfusion. 51 (9): 1883–85. doi:10.1111/j.1537-2995.2011.03270.x. PMID 21790637.
- Manco, Melania; Fernandez-Real, Josè (1 January 2012). "Back to past leeches: repeated phlebotomies and cardiovascular risk". BMC Medicine. 10 (1): 53. doi:10.1186/1741-7015-10-53. PMC 3409018. PMID 22647488.

- "Blood Plasma Safety" (PDF). GAO. Archived (PDF) from the original on 2008-06-25. Retrieved 2008-06-01.
- "Donate Blood For Money". Archived from the original on 2014-12-18.
- "Frequently Asked Questions". Australian Red Cross Blood Service. Archived from the original on 2011-07-22. Retrieved 2011-07-18.
- L. Fusco "From Latin America to Asia, Rising Above Difficulties, Achieving New Heights" AABB News. April 1998: p. 30
- "Guidelines for Implementation of Employee Blood Donation Leave" (PDF). New York State Department of Labor. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- "Legge 21 ottobre 2005, n. 219 (Law 21 October 2005, n. 219)". Italian Parliament. Archived from the original on 27 April 2009. Retrieved 2009-09-04.
- "Incentives program for blood donors and organizers". American Red Cross Connecticut Blood Services Region. Archived from the original on 2008-06-02. Retrieved 2008-06-01.
- "Red Cross Honour Roll". Archived from the original on 2012-09-28.
- "Archived copy". Archived from the original on 2013-02-04. Retrieved 2012-09-12.CS1 maint: archived copy as title (link)
- Steele WR; Schreiber GB; Guiltinan A; et al. (Jan 2008). "The role of altruistic behavior, empathetic concern, and social responsibility motivation in blood donation behavior". Transfusion. 48 (1): 43–54. doi:10.1111/j.1537-2995.2007.01481.x. PMID 17894795.
- "Richard Titmuss". National University of Taipei social work department. Archived from the original on 2011-01-15. Retrieved 2010-11-07.
- Catherine Waldby, The University of Sydney. "The logistics of altruism". Archived from the original on 2011-01-21. Retrieved 2010-11-07.
- Philippe Steiner. "Gifts of Blood and Organs : the Market and "Fictitious" Commodities". Revue française de sociologie 5/2003 (Volume 44), pp. 147–62. Archived from the original on 2011-12-04. Retrieved 2010-11-07.
Further reading
| Wikimedia Commons has media related to Blood donation. |
- Blood Donation and Processing
- How youths are supporting on blood emergency in Nepal?
- Deferred Donors: Anemia & Blood Donation
- British guidelines for transfusion medicine
- Definitive guide for safe blood donation.
- Harrison E (Nov 2007). "Blood cells for sale". Sci. Am. 297 (5): 108–9. doi:10.1038/scientificamerican1107-108. PMID 17990831.
(subtitle) There's more to blood banking than just bagging blood