Besifloxacin
Besifloxacin (INN/USAN) is a fourth-generation fluoroquinolone antibiotic. The marketed compound is besifloxacin hydrochloride. It was developed by SSP Co. Ltd., Japan, and designated SS734. SSP licensed U.S. and European rights to SS734 for ophthalmic use to InSite Vision Incorporated (OTC Pink: INSV) in 2000. InSite Vision developed an eye drop formulation (ISV-403) and conducted preliminary clinical trials before selling the product and all rights to Bausch & Lomb in 2003.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Besivance |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610011 |
| License data |
|
| Routes of administration | Ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
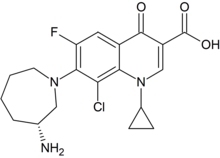
| Formula | C19H21ClFN3O3 |
| Molar mass | 393.84 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The eye drop was approved by the United States Food and Drug Administration (FDA) on May 29, 2009, and marketed under the trade name Besivance.[2]
Pharmacodynamics
Besifloxacin is a fluoroquinolone that has a broad spectrum in vitro activity against a wide range of Gram-positive and Gram-negative ocular pathogens: e.g., Corynebacterium pseudodiphtheriticum, Moraxella lacunata, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis, Streptococcus mitis, Streptococcus oralis, Streptococcus pneumoniae and Streptococcus salivarius. Besifloxacin has been found to inhibit production of pro-inflammatory cytokines in vitro.[3] The mechanism of action of besifloxacin involves inhibition of two enzymes which are essential for the synthesis and replication of bacterial DNA: the bacterial DNA gyrase and topoisomerase IV.
Medical use
Besifloxacin is indicated in the treatment of bacterial conjunctivitis caused by sensitive germs,[4] as well as in the prevention of infectious complications in patients undergoing laser therapy for the treatment of cataracts.[5][6]
Adverse effects
During the treatment, the most frequently reported ocular adverse reaction was the appearance of conjunctival redness (approximately 2% of patients). Other possible adverse reactions, reported in subjects treated with besifloxacin were: eye pain, itching of the eye, blurred vision, swelling of the eye or eyelid.
References
- "InSite Vision Reaches Agreement to Sell ISV-403 to Bausch & Lomb" (Press release). InSite Vision. 2003-12-19. Retrieved 2009-08-15.
- "Bausch & Lomb Receives FDA Approval of Besivance, New Topical Ophthalmic Antibacterial for the Treatment of Bacterial Conjunctivitis ("Pink Eye")" (Press release). Bausch & Lomb. 2009-05-29. Archived from the original on 2009-06-01. Retrieved 2009-05-29.
- Zhang JZ, Ward KW (January 2008). "Besifloxacin, a novel fluoroquinolone antimicrobial agent, exhibits potent inhibition of pro-inflammatory cytokines in human THP-1 monocytes". J. Antimicrob. Chemother. 61 (1): 111–6. doi:10.1093/jac/dkm398. PMID 17965029.
- Malhotra R, Ackerman S, Gearinger LS, Morris TW, Allaire C (December 2013). "The safety of besifloxacin ophthalmic suspension 0.6 % used three times daily for 7 days in the treatment of bacterial conjunctivitis". Drugs in R&D. 13 (4): 243–52. doi:10.1007/s40268-013-0029-1. PMC 3851703. PMID 24142473.
- Majmudar PA, Clinch TE (May 2014). "Safety of besifloxacin ophthalmic suspension 0.6% in cataract and LASIK surgery patients". Cornea. 33 (5): 457–62. doi:10.1097/ICO.0000000000000098. PMC 4195578. PMID 24637269.
- Nielsen SA, McDonald MB, Majmudar PA (2013). "Safety of besifloxacin ophthalmic suspension 0.6% in refractive surgery: a retrospective chart review of post-LASIK patients". Clinical Ophthalmology. 7: 149–56. doi:10.2147/OPTH.S38279. PMC 3552478. PMID 23355771.