Rifabutin
Rifabutin (Rfb) is an antibiotic used to treat tuberculosis and prevent and treat Mycobacterium avium complex.[1] It is typically only used in those who cannot tolerate rifampin such as people with HIV/AIDS on antiretrovirals.[1] For active tuberculosis it is used with other antimycobacterial medications.[1] For latent tuberculosis it may be used by itself when the exposure was with drug-resistant TB.[1]
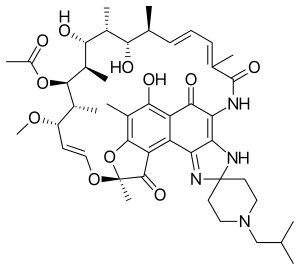 | |
| Clinical data | |
|---|---|
| Trade names | Mycobutin[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693009 |
| Pregnancy category | |
| Routes of administration | by mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 85% |
| Metabolism | liver |
| Elimination half-life | 28 to 62 hours (mean) |
| Excretion | kidney and fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.133.627 |
| Chemical and physical data | |
| Formula | C46H62N4O11 |
| Molar mass | 847.005 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include abdominal pain, nausea, rash, headache, and low blood neutrophil levels.[1] Other side effects include muscles pains and uveitis.[1] While no harms have been found during pregnancy it has not been well studied in this population.[1] Rifabutin is in the rifamycin family of medications.[1] Its mechanism of action is not clear.[1]
Rifabutin was approved for medical use in the United States in 1992.[1] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[2] The wholesale cost in the developing world is about US$30 a month.[3] In the United States a month of treatment is more than $200.[4]
Medical uses
Rifabutin is now recommended as first-line treatment for tuberculosis,[5] but rifampicin was used more widely because of its cheaper cost. However, due to the expiration of patents, prices are now similar.
Rifabutin is used in the treatment of Mycobacterium avium complex disease, a bacterial infection most commonly encountered in people with late-stage AIDS. Its main usefulness lies in the fact that it has fewer drug interactions than rifampicin; therefore people with HIV/AIDS on HAART are usually given rifabutin for treatment of TB.
Rifabutin is well tolerated in people with HIV-related tuberculosis (TB), but new findings suggest that with low CD4 cell counts have a high risk of treatment failure or relapse due to acquired rifamycin resistance. Since patients co-infected with TB and HIV/AIDS are likely to get TB treated first, when the CD4 is suppressed at the time TB treatment begins, doctors and patients should be aware of a possible rifamycin resistance.
Rifabutin is also being investigated in trials for treating Crohn's disease as part of the anti-MAP therapy. In a Phase III study administering sub-therapeutic doses of rifabutin in combination therapy to patients not identified with MAP infections, it was associated with significant short term benefits.[6][7]
It has also found to be useful in the treatment of Chlamydophila pneumoniae (Cpn) infection.
History
Scientists at the Italian drug company Achifar discovered rifabutin in 1975. (Eventually Archifar became part of Farmitalia Carlo Erba, a unit of the conglomerate Montedison which was subsequently bought by Pharmacia) This company's Adria Laboratories subsidiary filed for Food and Drug Administration (FDA) approval of rifabutin under the brand name Mycobutin in the early 1990s and the drug gained FDA approval in December 1992.
Rifabutin is primarily bactericidal antibiotic drug used to treat tuberculosis. Its effect on bacteria is based on the DNA-dependent RNA polymerase blocking drug rifamycin S, a semi-synthetic derivative. It is effective, for example, in highly resistant mycobacteria, Gram-positive bacteria (and some are effective against Gram-negative bacteria), but also against Mycobacterium tuberculosis, M. leprae, and M. avium intracellulare.
References
- "Rifabutin". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- "Rifabutin". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 50. ISBN 9781284057560.
- Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008 (WHO/HTM/TB/2008.402). Geneva, Switzerland: World Health Organization. 2008. p. ix. ISBN 978-92-4-154758-1. Archived from the original on 2008-10-18.
- "Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease". Gastroenterology. 132 (7): 2313–9. June 2007. doi:10.1053/j.gastro.2007.03.031. PMID 17570206.
- Kuenstner, J. Todd (2007). "The Australian Antibiotic Trial in Crohn's Disease: Alternative Conclusions From the Same Study". Gastroenterology. 133 (5): 1742–1743. doi:10.1053/j.gastro.2007.09.012.