Amlodipine/valsartan
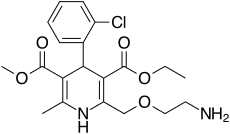
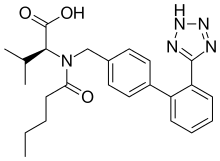
Amlodipine/valsartan is a blood pressure lowering combination drug. It contains amlodipine, a dihydropyridine-type calcium channel blocker, and valsartan, an angiotensin receptor blocker. This combination is usually well tolerated and effective for the reduction of blood pressure.[1]
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Valsartan | Angiotensin II receptor antagonist |
| Clinical data | |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| (verify) | |
The combination was originally patented by Novartis Pharmaceuticals under the brand name Exforge. The patent expired in September 2012.[2]
Amlodipine/valsartan is available in different dose preparations: 5 mg/80 mg, 5 mg/160 mg, 10 mg/160 mg, 5 mg/320 mg and 10/320 mg of amlodipine and valsartan, respectively.
The combination is also available with included hydrochlorothiazide, for patients who need a three-drug regimen to manage their blood pressure.
References
- Eckert, Siegfried; Freytag, Siegfried B.; Müller, Alfons; Klebs, Sven H. G. (2013). "Meta-analysis of three observational studies of amlodipine/valsartan in hypertensive patients with additional risk factors". Blood Pressure. 22 (sup1): 11–21. doi:10.3109/08037051.2013.793891. ISSN 0803-7051.
- "Generic Exforge HCT". Blood-pressure.emedtv.com. Retrieved 2012-03-05.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.