ST-segment elevation myocardial infarction (STEMI)
Contents
Background
- RV infarction accompanies ~25% of inferior STEMIs
- Hemodynamically significant only 10% of the time
- Do NOT reduce preload (NG, etc)
- Posterior (aka inferolateral) infarction is rarely isolated (~3-8% of all AMIs)
- Treat as STEMI
- Look for reciprocal changes, except in aVR and V1
- Apply V7, V8, V9 leads and repeat ECG looking for ST elevation
- Usually will see changes in V6 OR II, III, aVF
ACS Anatomical Correlation Chart
| Ischemic Changes | Location | Coronary Artery |
| STE V1-V3, TWI Q waves in V1-V3 over time |
Septal | Septal branch |
| STE V2-V4 | Anterior | LAD |
| STE I, aVL, V5, V6 STD inf leads |
Lateral | Circumflex |
| STE I, aVL, V2-6 | Anterolateral | LAD + circumflex = Left main or 2 critical lesions |
| STE II, III, aVF STD in aVL (most common lead to see reciprocal change) |
Inferior | RCA |
|
STE V1 (only lead looking at RV)
|
Right ventricle | RCA |
|
STD in V1, V2, V3; |
Posterior aka Inferolateral | RCA (90%), LCA (10%) |
| STE avR>V1 Doesn't apply in SVT |
Anterolateral | Left Main |
Prehospital
- Refrain from oxygen therapy if patient is hypoxic as hyperoxia my increase myocardial injury[1]
- Patients with a STEMI on the prehospital ECG but resolution of ST elevations on arrival still require activation of the cath team or transfer for primary catheterization even though there has been resolution of the ST-elevations[2]
Clinical Features
Risk of ACS
Clinical factors that increase likelihood of ACS/AMI:[3][4]
- Chest pain radiating both arms >R arm >L arm
- Chest pain associated with diaphoresis
- Chest pain associated with nausea/vomiting
- Chest pain with exertion
Clinical factors that decrease likelihood of ACS/AMI:[5]
- Pleuritic chest pain
- Positional chest pain
- Sharp, stabbing chest pain
- Chest pain reproducible with palpation
Male and female patients typical present with similar symptoms[6]
Differential Diagnosis
ST Elevation
- Myocardial infarct (STEMI)
- Post-MI (ventricular aneurysm pattern)
- Previous MI with recurrent ischemia in same area
- Wellens' syndrome
- Coronary artery vasospasm (eg, Prinzmetal's angina)
- Coronary artery dissection
- Drugs of abuse (eg, cocaine, crack, meth)
- Pericarditis
- Myocarditis
- Aortic dissection in to coronary
- LV aneurysm
- Early repolarization
- Left bundle branch block
- Left ventricular hypertrophy (LVH)
- Pneumomediastinum
- Pneumothorax
- Pulmonary Embolism
- Myocardial tumor
- Myocardial trauma
- External compression of artery
- Medications: Tricyclic (TCA) toxicity, Digoxin
- RV pacing (appears as Left bundle branch block)
- Hyperkalemia (only leads V1 and V2)
- Hypothermia ("Osborn J waves")
- Brugada syndrome
- Takotsubo cardiomyopathy
- AVR ST elevation
Evaluation
- When possible, comparison to old ECGs should be performed
- Repeating ECGs will increase sensitivity
- Look for ST segment elevation, hyperacute T waves, and ST depression in reciprocal leads
- Q waves are usually late, but may be early
- Usually 1/3 amplitude of QRS
- Use the J-point for measurement in 2 contiguous leads[7]
- J point is where there is a sudden change in direction
When to Activate Cath Lab
Classic STEMI
- Men
- Women
- ≥1.5 mm in V2-V3 and 1 mm (0.1mV) in all other leads[8]
Posterior STEMI
- Up to 10% of STEMIs are posterior
- Usually associated with infeior MI
- ≥0.5 mm STE is diagnostic
- Look at V1-V3[9][10]
- Large R waves (posterior Q waves)
- STD
- Upright T waves
Post-arrest STEMI/NSTEMI
- Get immediate ECG s/p arrest
- STEMI: go to cath lab immediately (AHA/ACCF Class IB)[11]
- NSTEMI: go to cath within 2 hrs if VT/VF, intractable ischemic pain, ADCHF (AHA/ACC Class IA)[12]
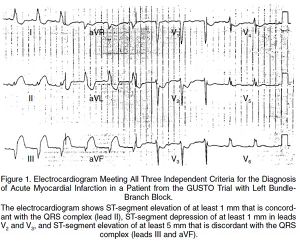
LBBB w/ Sgarbossa Criteria
- New LBBB alone is no longer STEMI criteria for cath lab as of 2013 per ACC/AHA guidelines[13]
- Hemodynamically unstable or new HF pts w/ new LBBB should be discussed with a cardiologist for PCI or fibrinolytics
- ≥3 points = 98% probability of STEMI[14]
- ST elevation ≥1 mm in a lead with upward (concordant) QRS complex - 5 points
- ST depression ≥1 mm in lead V1, V2, or V3 - 3 points
- ST elevation ≥5 mm in a lead with downward (discordant) QRS complex - 2 points
- Least specific of criteria, see Smith's modification
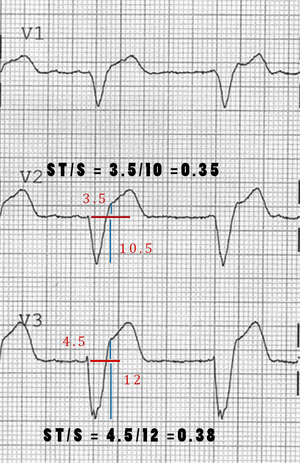
- Changes the 3rd rule of original Sgarbossa's Criteria to be ST depression OR elevation discordant w/ the QRS complex and w/ a magnitude of at least 25% of the QRS increases Sn from 52% to 91% at the expense of reducing Sp from 98% to 90%[15]
Consider Cath Lab Activation
- Pacemakers in AMI
- DeWinter T-waves[18]
- STE in aVR[21]
- Reflects subendocardial ischemia in LV (L main vs multi vessal disease)[22]
- Look for STE >1-1.5 mm in aVR
- Can also bee seen in hemorrhage, type A Dissection, massive PE
STEMI vs Pericarditis
| Disease | STEMI | Pericarditis |
| Pain | Costant | Varies with motion |
| Fever | No | Yes |
| ST changes | focal | Diffuse elevation |
| Reciprocal changes | Yes | No |
| Q waves | Yes | No |
| Pulmonary edema | Sometimes | No |
| Wall motion | Abnormal | Normal |
Management
Defibrillation
- Get the defibrillator ready early
- Mortality in patients with vfib is a function of time[23]
Adjunctive
- Aspirin 162-325mg chewable or 600mg PR
- Nitroglycerin
- Do not give if RV MI
- Do not give with phosphodiesterase inhibitors
- Morphine
- Beta-Blocker:
- PO within 24 hours
- Options[24]
- Acute MI: Metoprolol 5mg IV q2 min for x3 doses, then PO metoprolol 50mg q6hrs for 2 days starting 15 min after last IV dose, followed by maintenance of 100mg bid
- Post-MI: Atenolol 5mg IV over 5 min, then repeat in 10 min, then PO atenolol 50mg q12hrs for 7 days post-MI
- IV beta-blocker is reasonable for patients who are hypertensive in the absence of:
- Heart failure
- Low cardiac output state
- Cardiogenic shock risk factors
- Age > 70yr, sys BP < 120, HR > 110 or <60,
- Conduction block (PR interval > 0.24s, 2nd or 3rd block)
- Active asthma
- O2
Antiplatelets
Clopidogrel
- See drug link for specific age, indication related dosages
- Generally, loading dose of 600 mg if PCI anticipated (otherwise give 300 mg)
Ticagrelor
- May significantly reduce mortality as compared to clopidogrel[26]
- 180mg loading dose, followed by 90mg BID
- Ticagrelor offers no added benefit in STEMI when given pre-hospital vs. in-hospital (ambulance vs. cath lab)[27]
GPIIB/IIIa Inhibitors
- Abciximab, Eptifibatide
- Defer to cardiologist
- Given right before PCI depending on specific institutional protocols
Anticoagulation
- Heparin (UFH)
- Bolus 60U/kg (max: 4000U) followed by 12U/kg/h (max: 1000U/h)
- Titrate to PTT 1.5-2.5 x control
- LMWH
- <75yo with serum creatinine < 2.5mg/dL (men) or < 2.0mg/dL (women):
- 30mg IV bolus followed by 1mg/kg SC q12h
- ≥ 75yo
- 0.75mg/kg SC q12h
- CrCl < 30 mL/min
- 1mg/kg SC qd
- <75yo with serum creatinine < 2.5mg/dL (men) or < 2.0mg/dL (women):
- Fondaparinux
- creatinine < 3.0mg/dL:
- 2.5mg IV bolus then 2.5mg SC qd started 24hr after bolus
- Monitor anti-Xa levels
- creatinine < 3.0mg/dL:
- Bivalirudin
- 0.75mg/kg IV bolus followed by 1.75mg/kg/h
- CrCl < 30 mL/min
- 0.75mg/kg IV bolus followed by 1.0mg/kg/h
Definitive
The most critical aspect of care is to ensure systems are in place to minimize time taken for reperfusion. Anyone presenting within 12 hours of symptoms onset should have attempted reperfusion for STEMI. Options include fibrinolytic therapy or PCI. PCI is preferred if possible and had been demonstrated to result in superior outcomes.
- Percutaneous coronary intervention
- Goal: PCI should be attempted if the procedure can be started within 120 minutes (faster than 90 minutes is the goal, the faster the better)
- If the PCI cannot be commenced within 120 minutes then fibrinolysis should be given to suitable candidates
- Fibrinolytics
- Goal: if it is determined that PCI cannot be performed within 120 minutes then fibrinolytics should be given, and they should be given within 30 minutes
- If receive fibrinolytics also give anticoagulants for minimum of 48hr, and preferable the length of the hospitalization
- Fibrinolytic treatment within 3hr resulted in >30 lives saved per 1000 patients
- 0.5-1% of patients suffer ICH
Cardiac Arrest and STEMI
- Consider therapeutic hypothermia cooling protocol for patients with documented cardiac arrest felt to be caused by lethal cardiac rhythm (e.g. ventricular fibrillation)
- Patients with cardiac arrest and ST elevation at any point, even if resolved, should still under go emergent coronary angiography[28]
Fibrinolysis
Alteplase (TPA)
Dosing:
- >67kg pt:
- Infuse 15mg IV over 1-2min; then 50mg over 30min; then 35mg over next 60min (i.e. 100mg over 1.5hr)
- ≤67kg pt:
- Infuse 15mg IV over 1-2min; then 0.75 mg/kg (max 50mg) over 30 min; then 0.5 mg/kg over 60min (max 35 mg)
Tenecteplase-TNKase
- Reconstitute 50 mg vial in 10 mL sterile water (5 mg/mL)
- < 60 kg = 30 mg IV push over 5 seconds
- 60-69 kg = 35 mg IV push over 5 seconds
- 70-79 kg = 40 mg IV push over 5 seconds
- 80-89 kg = 45 mg IV push over 5 seconds
- > 90 kg = 50 mg IV push over 5 seconds
Indications
- <12hr from onset of CP
- ST elevation of ≥1mm in 2 contiguous limb or precordial leads OR new LBBB
- No PCI available within 90 min
- Should be able to see ST elevation in RBBB
Contraindications
Absolute contraindications
- Any prior ICH
- Known structural cerebral vascular lesion (AVM)
- Known intracranial neoplasm
- Ischemic stroke within 3 mo
- Active internal bleeding (excluding menses)
- Suspected aortic dissection or pericarditis
Relative contraindications
- Severe uncontrolled BP (>180/110)
- History of chronic severe poorly controlled hypertension
- History of prior ischemic stroke >3 mo
- Known intracranial pathology not covered in absolute contraindications
- Current use of anticoagulants with known INR >2–3
- Known bleeding diathesis
- Recent trauma (past 2 wk)
- Prolonged CPR (>10 min)
- Major surgery (<3 wk)
- Noncompressible vascular punctures (e.g. IJ, subclavian)
- Recent internal bleeding (within 2–4 wk)
- Patients treated previously with streptokinase should not receive streptokinase a 2nd time
- Pregnancy
- Active peptic ulcer disease
- Other medical conditions likely to increase risk of bleeding (diabetic retinopathy, etc)
Rescue PCI
- Failed reperfusion: consider if repeat ECG 90 minutes after infusion fails to show reduction of elevated ST segments by 50%
- Recurrent significant ST elevation following successful lysis
- Persistent hemodynamically unstable arrythmias, persistent ischemic symptoms, or worsened cardiogenic shock
- Even in those with successful reperfusion, its reasonable to do angiography within the index hospitalization, even within hours of thrombolytic therapy
Disposition
- Admit direct to cath lab
- If not at tertiary care center consider tPA depending transfer time and transfer to cardiac cath lab center
See Also
- Acute Coronary Syndrome (Main)
- NSTEMI
- ACS - Anatomical Correlation
- Myocardial Infarction Complications
- ST segment elevation
- Sgarbossa's criteria
- STEMI equivalents
- STEMI mimics
External Links
- MDCalc - TIMI Risk Score for STEMI
- GRACE score - ACS risk model
- STEMI heart attack
- ACC-AHA guidelines for STEMI 2013
- EMCrit LBBB
References
- ↑ 1.0 1.1 Stub D et al. Air versus oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation. 2015;121:2143-2150
- ↑ Ownbey M, Suffoletto B, Firsch A, et al. Prevalence and interventional outcomes of patients with resolution of ST-segment elevation between prehospital and in-hospital ECG. Prehosp Emerg Care. 2014. Apr-Jun;18(2):174-9
- ↑ Body R, Carley S, Wibberley C, et al. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation. 2010;81(3):281–286. PMID: 20036454
- ↑ Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280(14):1256–1263. PMID: 9786377
- ↑ Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294(20):2623–2629. PMID: 16304077
- ↑ Gimenez MR, et al. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med. 2014; 174(2):241-249.
- ↑ ACCF/AHA 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Jan 29;61(4):e78-140. PDF
- ↑ 8.0 8.1 8.2 Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction.. Third universal definition of myocardial infarction. Glob Heart. 2012 Dec;7(4):275-95
- ↑ Ayer A, Terkelsen CJ. Difficult ECGs in STEMI: lessons learned from serial sampling of pre- and in-hospital ECGs. Journal of Electrocardiology 2014;47(4):448–58
- ↑ Wei EY, Hira RS, Huang HD, et al. Pitfalls in diagnosing ST elevation among patients with acute myocardial infarction. Journal of Electrocardiology 2013;46(6):653–9
- ↑ O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. JAC 2013;61(4):e78–e140.
- ↑ Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64(24):e139–228.
- ↑ Am Heart J 2013;166:409-13
- ↑ Sgarbossa E. et al.. "Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators". NEJM. 1996. 334(8):481-7
- ↑ Smith, S. et al. Diagnosis of ST-Elevation Myocardial Infarction in the Presence of Left Bundle Branch Block With the ST-Elevation to S-Wave Ratio in a Modified Sgarbossa Rule. 60(6). 766-776
- ↑ Sgarbossa EB, Pinski SL, Gates KB, et al. Early electrocardiographic diagnosis of acute myocardial infarction in the presence of ventricular paced rhythm. GUSTO-I investigators. Am J Cardiol. 1996;77(5):423–424.
- ↑ Maloy KR, Bhat R, Davis J, et al. Sgarbossa Criteria are Highly Specific for Acute Myocardial Infarction with Pacemakers.West J Emerg Med. 2010;11(4):354–357.
- ↑ Birnbaum I, Birnbaum Y. High-risk ECG patterns in ACS—need for guideline revision. J Electrocardiol. 2013;46(6):535–9.
- ↑ Verouden NJ, Koch KT, Peters RJ, et al. Persistent precordial “hyperacute” T-waves signify proximal left anterior descending artery occlusion.
- ↑ de Winter RJ, Verouden NJ, Wellens HJ, et al. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008 Nov 6;359(19):2071-3.
- ↑ Rokos IC, French WJ, Mattu A, et al. Appropriate cardiac cath lab activation: optimizing the electrocardiogram interpretation and clinical decision making for acute ST-elevation myocardial infarction. Am Heart J. 2010 Dec; 160(6):995-1003.
- ↑ Kosuge M, Uchida K, Imoto K, et al. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2015 Jun 16;65(23):2570-1
- ↑ Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, Moss AJ. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004 Mar 9;109(9):1082-4.
- ↑ McAuley DF. Beta Blockers. GlobalRPH. http://www.globalrph.com/beta.htm
- ↑ Stub et al. Air Versus Oxygen in ST-Segment Elevation Myocardial Infarction. Circulation. 2015 May 22.
- ↑ Wallentin et Al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2009; 361:1045-1057.
- ↑ Montalescot G et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 2014 Sep 1.
- ↑ 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science PDF