Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission — United States and Kentucky, 2011–2014
Weekly / July 22, 2016 / 65(28);705–710
Alaya Koneru, MPH1; Noele Nelson, MD1; Susan Hariri, PhD1; Lauren Canary, MPH1; Kathy J. Sanders, MSN2; Justine F. Maxwell, MPH2; Xiaohua Huang, MS3; John A.D. Leake, MD3; John W. Ward, MD1; Claudia Vellozzi, MD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Illicit injection drug use is a risk factor for hepatitis C virus (HCV) infection; recent increases in injection drug use and increases in incidence of HCV infection among young persons have been observed in the United States.
What is added by this report?
During 2011–2014, increased rates of HCV detection (antibody or RNA positivity) among women of childbearing age and HCV testing (antibody or RNA) among children aged ≤2 years were observed both nationally and for Kentucky (22% and 14%, nationally; >200% and 151%, Kentucky). During the same period, birth certificate data showed the proportion of infants born to HCV-infected mothers increased 68% nationally and 124% in Kentucky.
What are the implications for public health practice?
Increased HCV testing of pregnant women, harmonized testing guidelines for children born to HCV-infected women, and adoption of a standardized perinatal HCV case definition might improve early identification of infants born to HCV-infected women and subsequent linkage of the mother and infant to care and treatment to prevent HCV-related sequelae.
Hepatitis C virus (HCV) infection is a leading cause of liver-related morbidity and mortality (1). Transmission of HCV is primarily via parenteral blood exposure, and HCV can be transmitted vertically from mother to child. Vertical transmission occurs in 5.8% (95% confidence interval = 4.2%–7.8%) of infants born to women who are infected only with HCV and in up to twice as many infants born to women who are also infected with human immunodeficiency virus (HIV) (2) or who have high HCV viral loads (3,4); there is currently no recommended intervention to prevent transmission of infection from mother to child (3). Increased reported incidence of HCV infection among persons aged ≤30 years (5,6) with similar increases among women and men in this age group (6), raises concern about increases in the number of pregnant women with HCV infection, and in the number of infants who could be exposed to HCV at birth. Data from one large commercial laboratory and birth certificate data were used to investigate trends in HCV detection among women of childbearing age,* HCV testing among children aged ≤2 years, and the proportions of infants born to HCV-infected women nationally and in Kentucky, the state with the highest incidence of acute HCV infection during 2011–2014 (6). During 2011–2014, commercial laboratory data indicated that national rates of HCV detection (antibody or RNA positivity†) among women of childbearing age increased 22%, and HCV testing (antibody or RNA) among children aged ≤2 years increased 14%; birth certificate data indicated that the proportion of infants born to HCV-infected mothers increased 68%, from 0.19% to 0.32%. During the same time in Kentucky, the HCV detection rate among women of childbearing age increased >200%, HCV testing among children aged ≤2 years increased 151%, and the proportion of infants born to HCV-infected women increased 124%, from 0.71% to 1.59%. Increases in the rate of HCV detection among women of childbearing age suggest a potential risk for vertical transmission of HCV. These findings highlight the importance of following current CDC recommendations to identify, counsel, and test persons at risk for HCV infection (1,7), including pregnant women, as well as consider developing public health policies for routine HCV testing of pregnant women, and expanding current policies for testing and monitoring children born to HCV-infected women. Expansion of HCV reporting and surveillance requirements will enhance case identification and prevention strategies.
In the United States, incidence of HCV infection has been increasing in young persons, including women of childbearing age, particularly in rural areas such as Appalachia (5,6). Although acute HCV infection, as defined by the Council of State and Territorial Epidemiologists,§ is a notifiable condition and reportable to the health department in almost all states,¶ persons with acute HCV infection account for a small fraction of persons with newly diagnosed HCV infection; most new diagnoses are among persons with HCV infection of unknown duration. Because reporting of all cases of HCV infection is not mandated in many states, a substantial proportion of HCV-infected women of childbearing age, including pregnant women, are likely not reported in routine state-based surveillance systems. Commercial laboratory data and birth certificate data provide additional sources of information to supplement HCV surveillance data.
To evaluate HCV infection among women of childbearing age and the potential for mother-to-child transmission of HCV, trends in HCV detection (defined as HCV antibody or RNA positivity) in women of childbearing age and HCV testing (antibody or RNA) among children aged ≤2 years from 2011–2014 were assessed nationally and for Kentucky using commercial laboratory data from Quest Diagnostics (Quest). Detection of HCV infection among infants was not evaluated because 1) the exact infant dates of birth to allow discrimination between maternal and infant HCV antibody were not available, and 2) very few infants had RNA testing to detect current HCV infection. Trends in proportions of infants born to HCV-infected women were assessed using birth certificate data from the National Center for Health Statistics. Maternal HCV infection status on birth certificates is obtained from the prenatal record, labor and delivery admission form, admission history and physical examination, or delivery record; maternal HCV diagnosis is recorded on the birth certificate if HCV infection is present at pregnancy diagnosis or if HCV infection is confirmed during pregnancy with a positive test for HCV (8). Demographic characteristics of HCV antibody-positive pregnant women reported to the Kentucky Department for Public Health (KDPH) during 2011–2014 were also examined. These data were collected as part of routine acute HCV surveillance, and during December 2013–December 2014, were enhanced by a KDPH request for voluntary reporting of all cases of HCV infection identified among pregnant women and infants.
The annual HCV detection rate among women of childbearing age tested by Quest was calculated as cases of HCV detection per 100,000 women of childbearing age served by the laboratory (i.e., women of childbearing age who received a laboratory test for any reason). Quest data were also used to calculate the annual HCV testing rate per 100,000 children aged ≤2 years served by Quest. The proportion of infants born to HCV-infected mothers was calculated using birth certificate data.
During 2011–2014, the national rate of HCV detection among women of childbearing age served by Quest increased 22%, from 139 to 169 per 100,000, and the rate of HCV testing among children aged ≤2 years served by Quest increased 14%, from 310 to 353 per 100,000 (Figure 1). During this time, the proportion of infants born to HCV-infected women nationally increased 68%, from one in 536 (0.19%) to one in 308 (0.32%) (Figure 2). During the same time, the rate of HCV detection among women of childbearing age in Kentucky increased 213%, from 275 to 862 per 100,000, and the rate of HCV testing among children aged ≤2 years increased 151%, from 403 to 1,011 per 100,000 (Figure 1). In addition, the proportion of infants born to HCV-infected women increased 124%, from one in 142 (0.71%) to one in 63 (1.59%) (Figure 2). During 2011–2014, HCV case reporting to KDPH identified 777 pregnant women with HCV antibody positivity; 527 (68%) were aged 20–29 years, 218 (28%) were aged 30–39 years, 653 (84%) were non-Hispanic white, and 293 (38%) reported past or current injection drug use.
Discussion
The national increases in HCV detection among women of childbearing age, HCV testing among infants, and the proportion of infants born to HCV-infected mothers suggest increased risk for mother-to-child transmission of HCV. This risk might be higher in certain areas of the United States, as illustrated by the findings in this report for Kentucky, which might be related to increasing illicit injection drug use (5). KDPH surveillance data for pregnant women are also consistent with demographic patterns of HCV incidence overall in Kentucky and nationally (6).
Many opportunities to improve identification and monitoring of HCV infection among women of childbearing age and infants exist. CDC recommends HCV testing for persons with a history of injection drug use and others at risk, including persons infected with HIV and persons with recognized exposures (e.g., health care workers after needle sticks or mucosal exposure to HCV-positive blood) (1,7). It is important that providers assess women of childbearing age, particularly pregnant women, for HCV risk and test accordingly. CDC also recommends HCV testing of children born to HCV-infected women (1,7). Several organizations have published guidelines on HCV testing of children,** but harmonization is needed to ensure that all women who are pregnant or planning pregnancy and all infants born to HCV-infected women are appropriately tested and linked to care if they are infected.
The potential for mother-to-child transmission of HCV has prompted some jurisdictions to consider changes in HCV case identification strategies and reporting policies. For example, the Philadelphia Department of Public Health recently demonstrated improved identification of infants born to HCV-infected mothers by cross-matching maternal information (including mother’s name and date of birth) on birth certificates to women in HCV surveillance registries (9). In 2015, Kentucky mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women.†† Development of national reporting criteria to include a case definition for perinatal HCV infection could standardize reporting across states. Reporting pregnancy status as part of HCV laboratory-based surveillance would also facilitate case identification. Improved surveillance can inform HCV screening and testing recommendations for pregnant women. Furthermore, there is an opportunity to detect HCV infection through routine HCV testing of infants identified as having perinatal exposure to illicit drugs, or neonatal abstinence syndrome, and their mothers; this could enhance HCV case identification as suggested by the large proportion of HCV antibody-positive pregnant women in Kentucky who report injecting illicit drugs.
The findings in this report are subject to at least four limitations. First, incomplete information on pregnancy status on case report forms used for surveillance in Kentucky and maternal HCV infection status on birth certificates might underestimate rates of infants born to HCV-infected mothers. Second, identifying cases of HCV-infected persons, including pregnant women, relies on completeness of reporting; therefore, the data from KDPH are likely underestimates. Third, laboratory data were limited to a single commercial laboratory and thus might not represent the United States and Kentucky populations. Finally, HCV-infected mothers cannot be linked to their children using laboratory data, and information on children’s age in the laboratory data are limited, making it difficult to determine whether children are appropriately tested and have current infection; thus, HCV detection rates among children aged ≤2 years were not included in this report.
These findings underscore the importance of providing primary prevention services (7) and following current recommendations to identify persons at risk for HCV infection and test accordingly; doing so among pregnant women would improve early identification of HCV-infected infants and linkage of the mother and infant to care and treatment. Furthermore, identifying HCV-infected women of childbearing age before pregnancy, with linkage to care, treatment, and cure, would avoid HCV infection during pregnancy and prevent mother-to-child transmission. Expanding current and developing new public health policies to increase HCV detection among women of childbearing age (especially pregnant women) and infants should be considered; however, additional data are needed to better assess HCV prevalence among pregnant women and their infants and investigate options for perinatal prevention, care, and treatment.
Acknowledgments
Shauna Onofrey, Dan Church, Massachusetts Department of Public Health; Danica Kuncio, Kendra Viner, Philadelphia Department of Public Health, Pennsylvania; Cecily Campbell, Division of Viral Hepatitis, CDC.
Corresponding author: Alaya Koneru, xjq8@cdc.gov, 404-639-0905.
1Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 2Kentucky Department for Public Health; 3Quest Diagnostics, Madison, New Jersey.
References
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012;61(No. RR-4). PubMed
- Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59:765–73. CrossRef PubMed
- Kanninen TT, Dieterich D, Asciutti S. HCV vertical transmission in pregnancy: new horizons in the era of DAAs. Hepatology 2015;62:1656–8. CrossRef PubMed
- Dunkelberg JC, Berkley EM, Thiel KW, Leslie KK. Hepatitis B and C in pregnancy: a review and recommendations for care. J Perinatol 2014;34:882–91. CrossRef PubMed
- Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015;64:453–8. PubMed
- CDC. Surveillance for viral hepatitis—United States, 2014. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. http://www.cdc.gov/hepatitis/statistics/2014surveillance/index.htm
- CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998;47(No. RR-19). PubMed
- National Center for Health Statistics. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision). Hyattsville, MD: National Center for Health Statistics; 2016. http://www.cdc.gov/nchs/data/dvs/guidetocompletefacilitywks.pdf
- Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis 2016;62:980–5. CrossRef PubMed
* Childbearing age among women is defined as 15–44 years.
† Quest Diagnostics detects antibody to HCV by an immunoassay, HCV RNA quantitatively by real-time polymerase chain reaction, and HCV RNA qualitatively by transcription mediated amplification.
§ Council of State and Territorial Epidemiologists. Hepatitis C, acute. https://wwwn.cdc.gov/nndss/conditions/hepatitis-c-acute.
¶ CDC. State reporting requirements for viral hepatitis. http://www.cdc.gov/hepatitis/featuredtopics/statereportingrequirements.htm.
** American Association for the Study of Liver Disease. HCV testing and linkage to care. http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. American Academy of Pediatrics. Hepatitis C. http://redbook.solutions.aap.org/chapter.aspx?sectionid=88187160&bookid=1484. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Diagnosis and management of hepatitis C infection in infants, children, and adolescents. http://www.naspghan.org/content/63/en/Clinical-Guidelines-and-Position-Statements.
†† Kentucky Reportable Disease Regulations: 902 Ky. Admin. Regs. 2:020.
 FIGURE 1. Hepatitis C virus (HCV) detection rate among females aged 15–44 years and HCV testing rate among children aged =2 years —United States and Kentucky, 2011–2014*
FIGURE 1. Hepatitis C virus (HCV) detection rate among females aged 15–44 years and HCV testing rate among children aged =2 years —United States and Kentucky, 2011–2014*
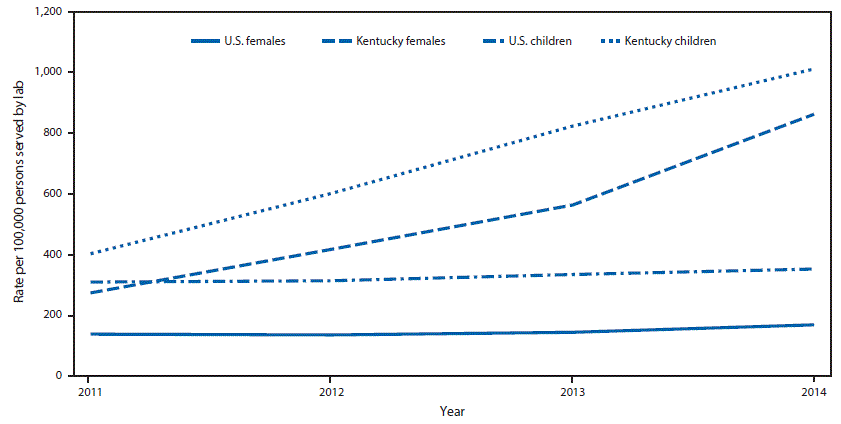
* HCV detection rates were calculated as number of females aged 15–44 years who received a positive HCV antibody and/or RNA result per 100,000 females aged 15–44 years served by Quest Diagnostics (i.e., received a laboratory test for any reason) by area of residence. HCV testing rates among children were calculated as number of children aged =2 years who received a test for HCV antibody and/or RNA per 100,000 children aged =2 years served by Quest Diagnostics by area of residence.
Source: Quest Diagnostics laboratory data.
 FIGURE 2. Proportion* of infants born to hepatitis C virus (HCV)-infected women† — United States and Kentucky, 2011–2014
FIGURE 2. Proportion* of infants born to hepatitis C virus (HCV)-infected women† — United States and Kentucky, 2011–2014
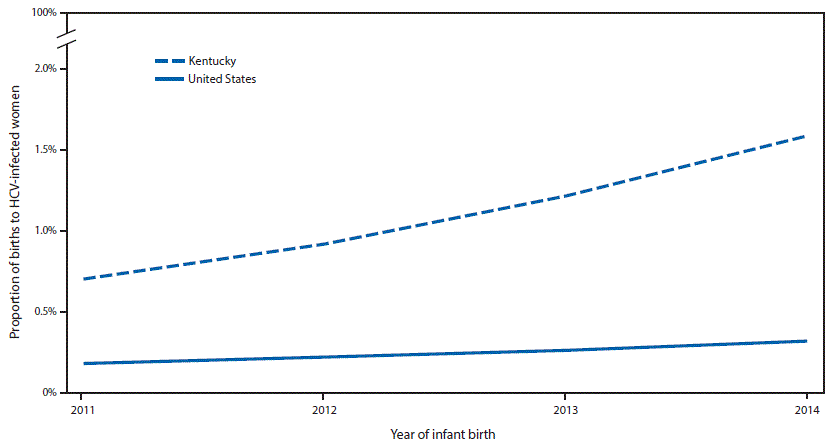
* Proportion calculated annually as infants born to HCV-infected women divided by total infants born.
† HCV infection status of mother is determined by notation on infant’s birth certificate. Birth categorization is based on mother’s place of residence.
Suggested citation for this article: Koneru A, Nelson N, Hariri S, et al. Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission — United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep 2016;65:705–710. DOI: http://dx.doi.org/10.15585/mmwr.mm6528a2.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 24, 2017
- Page last updated: August 24, 2017
- Content source:


 ShareCompartir
ShareCompartir