Possible Zika Virus Infection Among Pregnant Women — United States and Territories, May 2016
Weekly / May 27, 2016 / 65(20)
On May 20, 2016, this report was posted online as an MMWR Early Release.
Please note: An erratum has been published for this report. To view the erratum, please click here.
Regina M. Simeone, MPH1; Carrie K. Shapiro-Mendoza, PhD2; Dana Meaney-Delman, MD3; Emily E. Petersen, MD2; Romeo R. Galang, MD4,5; Titilope Oduyebo, MD2,4; Brenda Rivera-Garcia, DVM6; Miguel Valencia-Prado, MD7; Kimberly B. Newsome, MPH1; Janice Pérez-Padilla, MPH8; Tonya R. Williams, PhD9; Matthew Biggerstaff, MPH10; Denise J. Jamieson, MD2; Margaret A. Honein, PhD1; Zika and Pregnancy Working Group (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Zika virus infection during pregnancy causes microcephaly and other serious brain abnormalities. However, the full range of outcomes of asymptomatic and symptomatic Zika virus infection during pregnancy are not yet well understood.
What is added by this report?
In February 2016, CDC, in collaboration with state, local, tribal, and territorial health departments, launched comprehensive surveillance systems to report and actively monitor pregnancies and congenital outcomes among symptomatic and asymptomatic women with laboratory evidence of possible Zika virus infection. As of May 12, 2016, there were 157 and 122 pregnant women with laboratory evidence of possible Zika virus infection residing in U.S. states and U.S. territories, respectively.
What are the implications for public health practice?
This report launches the weekly reporting of pregnant women with laboratory evidence of possible Zika virus infection in U.S. states and territories. Monitoring all pregnant women with possible Zika virus infection during pregnancy, whether asymptomatic or symptomatic, will enhance understanding of possible adverse outcomes and allow better estimates of the number of pregnancies at risk for adverse outcomes. This information will assist health care providers who counsel pregnant women and will facilitate planning services for affected families.
Zika virus is a cause of microcephaly and brain abnormalities (1), and it is the first known mosquito-borne infection to cause congenital anomalies in humans. The establishment of a comprehensive surveillance system to monitor pregnant women with Zika virus infection will provide data to further elucidate the full range of potential outcomes for fetuses and infants of mothers with asymptomatic and symptomatic Zika virus infection during pregnancy. In February 2016, Zika virus disease and congenital Zika virus infections became nationally notifiable conditions in the United States (2). Cases in pregnant women with laboratory evidence of Zika virus infection who have either 1) symptomatic infection or 2) asymptomatic infection with diagnosed complications of pregnancy can be reported as cases of Zika virus disease to ArboNET* (2), CDC’s national arboviral diseases surveillance system. Under existing interim guidelines from the Council for State and Territorial Epidemiologists (CSTE), asymptomatic Zika virus infections in pregnant women who do not have known pregnancy complications are not reportable. ArboNET does not currently include pregnancy surveillance information (e.g., gestational age or pregnancy exposures) or pregnancy outcomes. To understand the full impact of infection on the fetus and neonate, other systems are needed for reporting and active monitoring of pregnant women with laboratory evidence of possible Zika virus infection during pregnancy. Thus, in collaboration with state, local, tribal, and territorial health departments, CDC established two surveillance systems to monitor pregnancies and congenital outcomes among women with laboratory evidence of Zika virus infection† in the United States and territories: 1) the U.S. Zika Pregnancy Registry (USZPR),§ which monitors pregnant women residing in U.S. states and all U.S. territories except Puerto Rico, and 2) the Zika Active Pregnancy Surveillance System (ZAPSS), which monitors pregnant women residing in Puerto Rico. As of May 12, 2016, the surveillance systems were monitoring 157 and 122 pregnant women with laboratory evidence of possible Zika virus infection from participating U.S. states and territories, respectively. Tracking and monitoring clinical presentation of Zika virus infection, all prenatal testing, and adverse consequences of Zika virus infection during pregnancy are critical to better characterize the risk for congenital infection, the performance of prenatal diagnostic testing, and the spectrum of adverse congenital outcomes. These data will improve clinical guidance, inform counseling messages for pregnant women, and facilitate planning for clinical and public health services for affected families.
Zika virus disease and congenital Zika virus infection are defined by the interim CSTE case definition and include confirmed and probable cases with laboratory evidence of infection (2). The clinical criteria for Zika virus disease include the presence of one of four symptoms (fever, rash, arthralgia, and conjunctivitis), or Guillain-Barré syndrome, or an adverse pregnancy outcome (fetal loss, or in utero findings of microcephaly or intracranial calcifications) in a symptomatic or asymptomatic mother with compatible illness or epidemiologic risk factors for Zika virus infection. Clinical criteria for Zika virus congenital infection in infants include microcephaly, intracranial calcifications, or other central nervous system abnormalities (2). Jurisdictions report cases meeting these criteria to ArboNET. Although jurisdictions can report asymptomatic infection in pregnant women without pregnancy complications to ArboNET, this reporting is at the discretion of the local jurisdiction and is not universal. Current ArboNET reporting includes cases of Zika virus disease that meet the interim CSTE case definition.
For the purposes of the USZPR and ZAPSS, laboratory evidence of possible Zika virus infection is defined as a positive Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) test result (i.e., a confirmed case of Zika virus infection) or an equivocal or presumptive positive Zika virus immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (ELISA) test result (3–5).¶ Plaque reduction neutralization testing (PRNT) performed in conjunction with the IgM ELISA must have Zika PRNT titers ≥10 for inclusion. Pregnant women who meet laboratory criteria are included in the surveillance systems whether they report symptoms or not. Women are included retrospectively if laboratory evidence of congenital Zika virus infection is identified in fetal tissues, the placenta, or the infant.
The USZPR was initiated primarily to monitor outcomes in pregnant women returning from travel to areas with local Zika virus transmission (6). To date the majority of cases in pregnant women reported to USZPR are associated with travel, but it also includes cases of sexual transmission (7) and local transmission from the U.S. territories. ZAPSS was developed separately for Puerto Rico to conduct enhanced surveillance in pregnant women at risk for Zika virus infection as a result of ongoing local Zika virus transmission. Using USZPR and ZAPSS, CDC will report the number of pregnant women with laboratory evidence of possible Zika virus infection weekly on its website. Data reported by noon Eastern Standard Time each Thursday (for this report, May 12, 2016) will be verified and reported in aggregate the following Thursday. Reporting is subject to a lag of 1 week to verify data from each participating jurisdiction. Reports from Arizona and Idaho have not yet been verified and are excluded from the current report.
As of May 12, 2016, combined data from USZPR and ZAPSS include 279 reports of pregnant women with laboratory evidence of possible Zika virus infection, including 157 pregnant women residing in U.S. states and the District of Columbia (Figure 1) and 122 residing in U.S. territories (Figure 2). As of May 11, 2016, 113 pregnant women meeting clinical criteria for Zika virus disease were reported to CDC through ArboNET, 48 in U.S. states, and 65 in U.S. territories.
Among the 157 pregnant women from U.S. states and the District of Columbia monitored through USZPR, 73 (49%)** reported clinical symptoms consistent with Zika virus disease. Among these symptomatic pregnant women, 64 (88%) reported rash, 36 (49%) arthralgia, 37 (51%) fever, and 17 (23%) conjunctivitis. Among all pregnancies included from U.S. states, Zika virus nucleic acid detection by rRT-PCR was reported in 39 (25%).
Among 122 pregnant residents of the U.S. territories†† being monitored in USZPR or ZAPSS, 80 (66%)§§ reported clinical symptoms consistent with Zika virus disease. Among these symptomatic women, 60 (75%) reported rash, 29 (36%) arthralgia, 27 (34%) fever, and 15 (19%) conjunctivitis. Among all women included from U.S. territories, Zika virus nucleic acid detection by rRT-PCR in serum was identified in 67 (55%).
Discussion
Through the establishment of these pregnancy surveillance systems, CDC, in collaboration with state, local, tribal, and territorial partners, is reporting and actively monitoring pregnant women with laboratory evidence of possible Zika virus infection. These surveillance systems monitor pregnant women at risk for adverse congenital outcomes attributable to possible Zika virus infection. Including pregnant women with laboratory evidence of possible Zika virus infection but without a reported history of symptoms more than doubles the number of pregnancies being monitored, compared with pregnancies meeting the interim CSTE case definition and reported by ArboNET.
Limiting surveillance to symptomatic women with confirmed or probable Zika virus disease or to women already affected by an adverse pregnancy outcome excludes a substantial proportion of women with asymptomatic and possible Zika virus infection during pregnancy. In contrast, the broader case definition used for the USZPR and ZAPSS surveillance systems might overestimate Zika virus infection among women screened for infection because of crossreactivity with dengue and other flaviviruses, particularly among residents of U.S. territories and travelers with a history of prior flavivirus infection or flavivirus vaccination (8), or nonspecific reactivity.
Case reports indicate that fetuses and infants of pregnant women with asymptomatic Zika virus infection might be at risk for microcephaly and other severe brain defects (9,10). Following pregnant women with laboratory evidence of possible Zika virus infection in the surveillance system, regardless of symptoms, allows better characterization of the full impact and consequences of infection to the mother and her offspring, and might allow for better stratification of risk for adverse congenital outcomes (1). An important role of the USZPR and ZAPSS surveillance systems is evaluating the range of outcomes associated with Zika virus infection during pregnancy. Pregnancy outcomes are currently being monitored and will be shared in future reports. It is critical that health care providers inform state, local, tribal, and territorial health departments of any pregnant women with laboratory evidence of possible Zika virus infection under their care.
The findings in this report are subject to at least three limitations. First, data provided to the jurisdictions and CDC regarding symptoms and symptom onset might not be accurate or complete because of variability in recall by patients or data available to jurisdictions. Second, only pregnant women who are tested for Zika virus infection are included, thereby potentially underestimating the prevalence of infection and outcomes among all pregnant women. Finally, all states are not included in the USZPR, possibly affecting the representativeness of these data with regard to all pregnant women identified with a possible Zika virus infection.
One challenge of this Zika virus outbreak is the lack of understanding of the magnitude of risk and spectrum of outcomes associated with Zika virus infection during pregnancy. The USZPR and ZAPSS are surveillance systems established to enumerate and describe pregnancies with Zika virus infection and risk for adverse outcomes associated with infection during pregnancy. Findings from these U.S. surveillance systems are expected to improve understanding of Zika virus infection during pregnancy, enhance risk assessment and counseling of pregnant women and families, advance clinical care, and assist states and territories to anticipate and plan needed resources and increase prevention efforts.¶¶
Acknowledgments
Joel Ackelsberg, New York City Department of Health and Mental Hygiene; Connie Austin, Illinois Department of Public Health; Ihsan Azzam, State of Nevada Department of Health and Human Services; Bryon Backenson, New York State Department of Health; Hayley D. Belisle-Yaglom, Arizona Department of Health Services; Sara Blosser, Indiana State Department of Health; John Bos, Missouri Department of Health and Senior Services; Kelly Broussard, Texas Department of State Health Services; Jen Brown, Indiana State Department of Health; Louisa Castrodale, Alaska Division of Public Health; Glenn Copeland, Michigan Department of Health and Human Services; Julie Coughlin, Iowa Department of Public Health; Laura Cronquist, North Dakota Department of Health; Alexander Davidson, New York City Department of Health and Mental Hygiene; John O. Davies-Cole, District of Columbia Department of Health; Mychal Davis, Kansas Department of Health and Environment; Stephanie Dearth, Indiana State Department of Health; Catherine Dentinger, New York City Department of Health and Mental Hygiene; Elizabeth Dufort, New York State Department of Health; Cherie Drenzek, Georgia Department of Public Health; Dan Drociuk, South Carolina Department of Health and Environmental Control; Esther Ellis, U.S. Virgin Islands Department of Health; Brenda Esponda-Morrison, Connecticut Department of Public Health; Nicole Evert, Texas Department of State Health Services; Shawna Feinman, Georgia Department of Public Health; Michelle Feist, North Dakota Department of Health; Annie Fine, New York City Department of Health and Mental Hygiene; Debbie Freeman, Illinois Department of Public Health; Kristin Garafalo, New Jersey Department of Health; Ann Garvey, Iowa Department of Public Health; Carla Grayson, Arkansas Department of Health; Jyoti Gupta, Virginia Department of Health; Christine G. Hahn, Idaho Department of Health and Welfare; Dirk Haselow, Arkansas Department of Health; Lea Heberlein-Larson, Florida Department of Health; Preetha J. Iyengar, District of Columbia Department of Health; Erin Jenkins, Maryland Department of Health and Mental Hygiene; Loletha Johnson, New Jersey Department of Health; Jenna Iberg Johnson, Louisiana Office of Public Health; Diep Hoang Johnson, Wisconsin Department Health Services; Mary Knapp, New Jersey Department of Health; Edward Lifshitz, New Jersey Department of Health; Anna M. Likos, Florida Department of Health; Judy Lovchik, Indiana State Department of Health; Kim Machesky, Ohio Department of Health; Emily McGibbon, New York City Department of Health and Mental Hygiene; Natasha McIntosh, New York City Department of Health and Mental Hygiene; Nancy Mimm, New Jersey Department of Health; Marika Mohr, Ohio Department of Health; Christine L. Mulgrew, State of Montana Department of Health and Human Services; Betsy Negron, Pennsylvania Department of Health; David Neitzel, Minnesota Department of Health; Randall Nelson, Connecticut Department of Public Health; Candace Noonan-Toly, New York State Department of Health; Kara McGinnis Pilote, CDC and New Jersey Department of Health; Pam Pontones, Indiana State Department of Health; Rachel Radcliffe, South Carolina Department of Health and Environmental Control; Jennifer L. Rakeman, New York City Department of Health and Mental Hygiene; Joy Rende, New Jersey Department of Health; Sara Robeson, Kentucky Department for Public Health; Angela M. Rohan, Wisconsin Department Health Services, CDC; Sarah Scotland, Massachusetts Department of Public Health; Nancy Scotto-Rosato, New Jersey Department of Health; Lylah Seaton, Florida Department of Health; Lori Simmons, Arkansas Department of Health; Theresa Sokol, Louisiana Office of Public Health; Lisa Sollot, Virginia Department of Health; Jamie Sommer, New York State Department of Health; Mary Grace Stobierski, Michigan Department of Health and Human Services; Christina Tan, New Jersey Department of Health; Anthony Tran, New York City Department of Health and Mental Hygiene; Emily Valencia, Virginia Department of Health; Warren Villagomez, Northern Mariana Islands Department Of Public Health; Sharon Watkins, Pennsylvania Department of Health; Christian Whelen, Hawaii Department of Health; Amie Worthington, Kansas Department of Health and Environment; Karen Worthington, New Jersey Department of Health; Bryan Buss, Martín Celaya, Tai-Ho Chen, Kenneth L. Dominguez, Divia Forbes, Jessica Goodell, Mary Goodwin, Thane Hancock, Theresa Harrington, Stacy Holzbauer, Tippavan Nagachinta, Alba Phippard, Kimberly Porter, Araceli Rey, Audilis Sanchez, Nicholas Somerville, Óscar Tarragó, CDC.
Corresponding author: Regina M. Simeone, eocbirthdef@cdc.gov, 770-488-7100.
1Division of Congenital and Developmental Disorders, National Center on Birth Defects and Developmental Disabilities, CDC; 2Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC; 3Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 4Epidemic Intelligence Service, CDC; 5Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and Tuberculosis Prevention, CDC; 6Office of Epidemiology and Research, Puerto Rico Department of Health; 7Puerto Rico Birth Defects Surveillance and Prevention System, Puerto Rico Department of Health; 8Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 9Division of Human Development and Disability, National Center on Birth Defects and Developmental Disabilities, CDC; 10Influenza Division, National Center for Immunization and Respiratory Diseases, CDC.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 2016;374:1981–7. CrossRef PubMed
- Council of State and Territorial Epidemiologists. Zika virus disease and congenital Zika virus infection interim case definition and addition to the Nationally Notifiable Diseases List. Position statement PS 16-ID-01 (interim). Atlanta, GA: Council of State and Territorial Epidemiologists; 2016. https://www.cste2.org/docs/Zika_Virus_Disease_and_Congenital_Zika_Virus_Infection_Interim.pdf
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:122–7. CrossRef PubMed
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:315–22. CrossRef PubMed
- CDC. Zika MAC-ELISA—for use under an emergency use authorization only. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM488044.pdf
- Meaney-Delman D, Hills SL, Williams C, et al. Zika virus infection among U.S. pregnant travelers—August 2015–February 2016. MMWR Morb Mortal Wkly Rep 2016;65:211–4. CrossRef PubMed
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—Continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:215–6. CrossRef PubMed
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med 2016;374:1552–63. CrossRef PubMed
- Sarno M, Sacramento GA, Khouri R, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis 2016;10:e0004517. CrossRef PubMed
- Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill 2016;21:22–30. CrossRef PubMed
* http://www.cdc.gov/westnile/resourcepages/survresources.html.
† In the surveillance systems, laboratory evidence of Zika virus infection is defined as a positive Zika virus real-time reverse transcription–polymerase chain reaction test or a positive Zika virus immunoglobulin M (IgM) antibody test using the CDC IgM antibody capture enzyme-linked immunosorbent assay (ELISA). Plaque reduction neutralization testing (PRNT) performed in conjunction with the IgM ELISA must have Zika PRNT titers ≥10.
§http://www.cdc.gov/zika/hc-providers/registry.html.
¶http://www.cdc.gov/zika/hc-providers/diagnostic.html; http://www.cdc.gov/zika/hc-providers/qa-pregnant-women.html.
** Eight missing information on symptom status.
†† All U.S. territories are participating.
§§ One missing information on symptom status.
 FIGURE 1. Week of illness onset for symptomatic pregnant women or specimen collection date* for asymptomatic pregnant women†,§ with laboratory evidence¶ of possible Zika virus infection, by symptom status (N = 142)** — 48 states†† and the District of Columbia, April 26, 2015–May 12, 2016
FIGURE 1. Week of illness onset for symptomatic pregnant women or specimen collection date* for asymptomatic pregnant women†,§ with laboratory evidence¶ of possible Zika virus infection, by symptom status (N = 142)** — 48 states†† and the District of Columbia, April 26, 2015–May 12, 2016
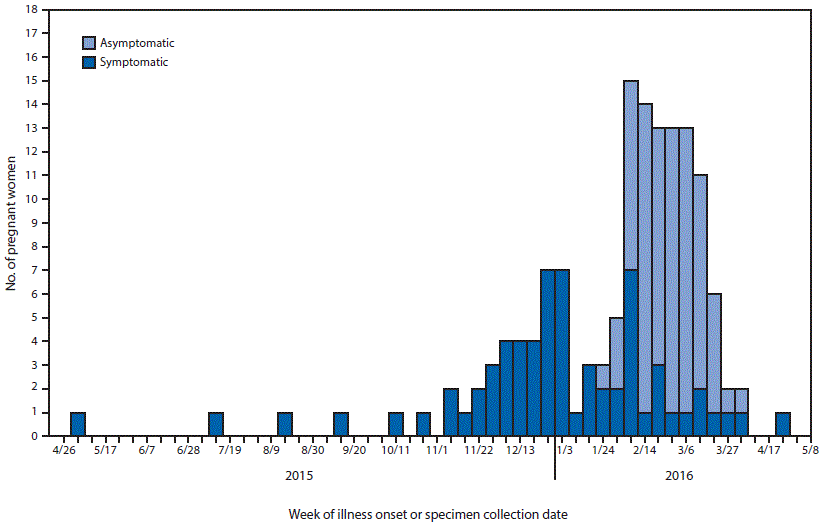
Abbreviations: ELISA = enzyme-linked immunosorbent assay; IgM = immunoglobulin M; PRNT = plaque reduction neutralization test.
* Date of onset of symptoms or testing.
† Specimen collection dates for asymptomatic pregnant women might not coincide with the period of exposure or infection with Zika virus.
§ CDC issued updated interim guidelines on February 5, 2016, to include recommending serologic testing of asymptomatic pregnant women 2–12 weeks after travel to an affected area.
¶ Laboratory evidence of possible Zika virus infection is defined as a positive Zika virus real-time reverse transcription–polymerase chain reaction test or a positive Zika virus IgM ELISA test; if PRNT is performed in conjunction with the IgM ELISA, Zika PRNT titers must be =10 for inclusion.
** Excludes 15 women with missing symptom status or missing date of symptom onset.
†† Figure includes data for U.S. states from the U.S. Zika Pregnancy Registry, excluding Arizona and Idaho.
 FIGURE 2. Week of illness onset for symptomatic pregnant women or specimen collection date* for asymptomatic pregnant women†,§ with laboratory evidence¶ of possible Zika virus infection, by symptom status (N = 115)** — U.S. territories,†† January 3, 2016–May 12, 2016
FIGURE 2. Week of illness onset for symptomatic pregnant women or specimen collection date* for asymptomatic pregnant women†,§ with laboratory evidence¶ of possible Zika virus infection, by symptom status (N = 115)** — U.S. territories,†† January 3, 2016–May 12, 2016
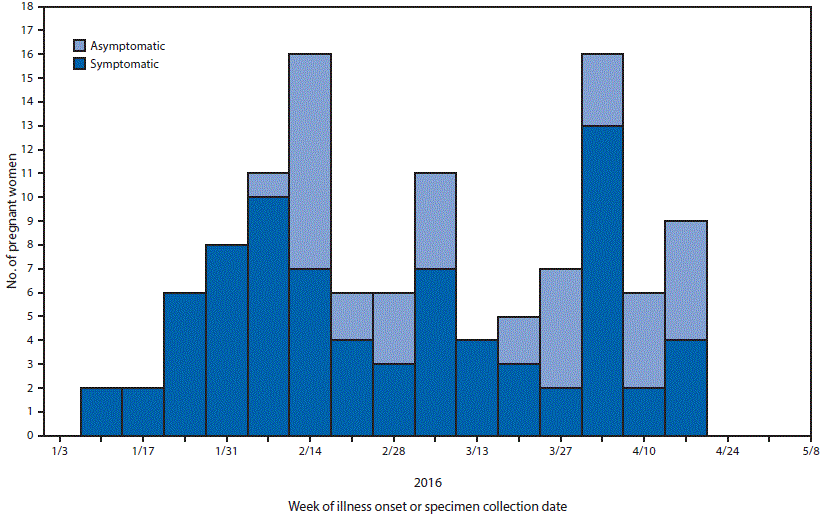
Abbreviations: ELISA = enzyme-linked immunosorbent assay; IgM = immunoglobulin M; PRNT = plaque reduction neutralization test.
* Date of onset of symptoms or testing.
† Specimen collection dates for asymptomatic pregnant women might not coincide with the period of exposure or infection with Zika virus.
§ CDC issued updated interim guidelines on February 5, 2016, to include recommending serologic testing of asymptomatic pregnant living in an area with active Zika virus transmission in the first and second trimester.
¶ Laboratory evidence of possible Zika virus infection is defined as a positive Zika virus real-time reverse transcription–polymerase chain reaction test or a positive Zika virus IgM ELISA test; if PRNT is performed in conjunction with the IgM ELISA, Zika PRNT titers must be =10 for inclusion.
** Excludes seven women with missing symptom status or missing date of symptom onset.
†† Figure includes data for U.S. territories from the U.S. Zika Pregnancy Registry and the Zika Active Pregnancy Surveillance System.
Zika and Pregnancy Working Group
Farah Ahmed, Kansas Department of Health and Environment; Scott Anesi, American Samoa Department of Health; Kathryn E. Arnold, CDC; Danielle Barradas, CDC; Devra Barter, Colorado Department of Public Health and Environment; Jeanne Bertolli, CDC; Andrea M. Bingham, Florida Department of Health; Jan Bollock, South Dakota Department of Health; Trish Bosse, Maine Department of Health and Human Services; Kristy K. Bradley, Oklahoma State Department of Health; Diane Brady, Rhode Island Department of Health; Catherine M. Brown, Massachusetts Department of Public Health; Katie Bryan, Wyoming Department of Health; Victoria Buchanan, Indiana State Department of Health; Ponce D. Bullard, South Carolina Department of Health and Environmental Control; Alice Carrigan, Maricopa County Department of Public Health; Monica Clouse, Kentucky Department for Public Health; Sally Cook, Vermont Department of Health; Michael Cooper, Alaska Division of Public Health; Sherri Davidson, Alabama Department of Health; Ariana DeBarr, West Virginia Bureau for Public Health; Thomas Dobbs, Mississippi Department of Health; Tambra Dunams, CDC; Jeffrey Eason, Utah Department of Health; Amanda Eckert, Houston Health Department; Paula Eggers, Delaware Division of Public Health; Sascha R. Ellington, CDC; Amanda Feldpausch, Georgia Department of Public Health; Carolyn R. Fredette, New Hampshire Department of Health and Human Services; Julie Gabel, Georgia Department of Public Health; Maleeka Glover, CDC; Michael Gosciminski, Rhode Island Department of Health; Margarita Gay, Guam Department of Public Health and Social Services; Robert Haddock, Guam Department of Public Health and Social Services; Sheryl Hand, Mississippi Department of Health; Jessica Hardy, Alabama Department of Health; Marie E. Bottomley Hartel, Tennessee Department of Health, Andrew; K. Hennenfent, CDC/CSTE Fellow and District of Columbia Department of Health; Susan L. Hills, CDC; Jennifer House, Colorado Department of Public Health and Environment; Iro Igbinosa, CDC; Lucy Im, Arkansas Department of Health; Hamik Jeff, Nebraska Department of Health and Human Services; Sumaiya Khan, CDC; Lon Kightlinger, South Dakota Department of Health; Jean Y. Ko, CDC; Samir Koirala, CDC and Nebraska Department of Health and Human Services; Lauren Korhonen, CDC; Vikram Krishnasamy, CDC; Katie Kurkjian, Virginia Department of Health; Margaret Lampe, CDC; Sandra Larson, State of Nevada Department of Health and Human Services; Ellen H. Lee, New York City Department of Health and Mental Hygiene; Leah Lind, Pennsylvania Department of Health; Scott Lindquist, Washington State Department of Health; Jonah Long, Pennsylvania Department of Health; Jennifer Macdonald, Virginia Department of Health; Jennifer MacFarquhar, CDC; Daniel P. Mackie, State of Nevada Department of Health and Human Services; Miguella Mark-Carew, West Virginia Bureau for Public Health; Brennan Martin, Vermont Department of Health; Alma Martinez-Quiñones, Puerto Rico Department of Health; Janice Matthews-Greer, Michigan Department of Health and Human Services; Sasha A. McGee, District of Columbia Department of Health; Joe McLaughlin, Alaska Division of Public Health; Valerie Mock, Florida Department of Health; Esther Muna, Northern Mariana Islands Department Of Public Health; Hanna Oltean, Washington State Department of Health; Josephine O’Mallan, Guam Department of Public Health and Social Services; H. Pamela Pagano, CDC; Sarah Y. Park, Hawaii Department of Health; Dallin Peterson, Utah Department of Health; Kara N.D. Polen, CDC; Charsey Cole Porse, California Department of Public Health; Carol Y. Rao, CDC; Abubakar Ropri, New Mexico Department of Health; Jessica Rinsky, CDC; Sara Robinson, Maine Department of Health and Human Services; Asher Y. Rosinger, CDC; Irene Ruberto, Arizona Department of Health Services; Elizabeth Schiffman, Minnesota Department of Health; Christine Scott-Waldron, Louisiana Office of Public Health; Shereen Semple, New Jersey Department of Health; Tyler Sharp, CDC; Kirstin Short, Houston Health Department; Kimberly Signs, Michigan Department of Health and Human Services; Sally A. Slavinski, New York City Department of Health and Mental Hygiene; Taryn Stevens, Indiana State Department of Health; Joseph Sweatlock, New Jersey Department of Health; Elizabeth A. Talbot, New Hampshire Department of Health and Human Services; Julius Tonzel, Louisiana Office of Public Health; Rita Traxler, CDC; Sheri Tubach, Kansas Department of Health and Environment; Clayton Van Houten, Wyoming Department of Health; Elizabeth VinHatton, New Mexico Department of Health; Melissa Viray, Hawaii Department of Health; Daguise Virginie, South Carolina Department of Health and Environmental Control; Michael D. Warren, Tennessee Department of Health; Catherine Waters, Arkansas Department of Health; Paul White, Northern Mariana Islands Department Of Public Health; Tanya Williams, CDC; Ann I. Winters, New York City Department of Health and Mental Hygiene; Shelley Wood, Kentucky Department for Public Health; Ibrahim Zaganjor, CDC; (all of these individuals meet collaborator criteria).
Suggested citation for this article: Simeone RM, Shapiro-Mendoza CK, Meaney-Delman D, et al. Possible Zika Virus Infection Among Pregnant Women — United States and Territories, May 2016. MMWR Morb Mortal Wkly Rep 2016;65. DOI: http://dx.doi.org/10.15585/mmwr.mm6520e1.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 29, 2017
- Page last updated: August 29, 2017
- Content source:


 ShareCompartir
ShareCompartir