Prevalence of Amyotrophic Lateral Sclerosis — United States, 2010-2011
Corresponding author: Paul Mehta, MD, Division of Toxicology and Human Health Sciences, CDC/ATSDR. Telephone: 770-488-0556; E-mail: pum4@cdc.gov.
MMWR in Brief summarizes key points from "Prevalence of Amyotrophic Lateral Sclerosis — United States, 2010-2011." MMWR 2014;63(No. SS-7). Available at http://www.cdc.gov/mmwr/pdf/ss/ss6307.pdf. Certain text might not have appeared in the original publication.
New Information
This is the first population-based prevalence estimate and description of demographic characteristics of amyotrophic lateral sclerosis (ALS) for the United States. Data originated from the Agency for Toxic Substances and Disease's National ALS Registry launched in 2009. Registry findings are consistent with estimates from long-established ALS registries in Europe and from smaller-scale epidemiologic studies conducted previously in the United States.
Analysis
The prevalence of ALS was calculated from the registry data. Demographic characteristics were described by sex, age, race, and ethnicity. The numerator was obtained by using the de-duplicated total number of persons with ALS identified through administrative data and those who self-identified through a secure web portal (Figure 1). The 2011 Census was used for the denominator. Although national incidence cannot be measured with registry data at this time, incidence is being measured in smaller geographic areas (three states and eight metropolitan areas) that have participated in ATSDR's State and Metropolitan Area ALS surveillance projects.
Summary Findings
During October 19, 2010-December 31, 2011, a total of 12,187 persons meeting the surveillance case definition for definite ALS were identified by the registry, for a prevalence of 3.9 cases per 100,000 persons in the U.S. general population (Figure 2). Overall among affected persons, ALS was more common among whites, males, non-Hispanics, and persons aged 60-69 years. By age group, the lowest number of ALS cases were in persons aged 18-39 years and >80 years. Males had a higher prevalence rate of ALS than females overall and across all data sources.
Data Sources and Methods
In 2008, the U. S. Congress passed the National Amyotrophic Lateral Sclerosis Registry Act (1) which allowed for the creation of a population-based registry to study ALS. In 2009, ATSDR launched the National Amyotrophic Lateral Sclerosis Registry. The purpose of the registry is to describe the incidence and prevalence of ALS in the United States, define the demographic characteristics of those with ALS, and examine risk factors that might be related to the development of ALS. Because ALS is not a notifiable disease in the United States, the registry uses novel approaches to identify ALS cases. These approaches include using administrative datasets (beginning in 2009) and a self-enrollment web portal (launched on October 19, 2010) (Figure 1). This is the first effort to estimate the prevalence of ALS at the national level.
The National Amyotrophic Lateral Sclerosis Registry is the first national registry to use existing administrative data (from Medicare, Medicaid, the Veterans Heath Administration [VHA], and the Veterans Benefits Administration [VBA]) as a major source of case ascertainment. An algorithm was used to identify persons with ALS based on health record encounters such as neurologist or other physician visits with an International Classification of Diseases Ninth Revision (ICD-9) codes 335.20 or a VBA code 8017, a prescription for Riluzole, and/or a death certificate listing ALS as a cause or contributing cause of death. Only data for persons categorized as "definite ALS" (positive diagnosis of ALS) were included in the report. Those categorized as "possible ALS" (diagnosis of ALS is not confirmed) are reevaluated as additional years of data become available. In addition, a secure web portal is used for self-registration of persons with ALS after a series of validation questions. Once registered, participants can complete modules about about potential risk factors.
Main Results
During October 19, 2010-December 31, 2011, a total of 12,187 cases were categorized as "definite ALS" across the four national databases and through web portal registration. The overall prevalence rate of ALS was 3.9 per 100,000 persons and increased as age increased. The rate was lowest among persons aged 18-39 years (0.5) and highest among persons aged 70-79 years (17.0) (Figure 2). When the self-enrollment portal data were analyzed alone, the rate was highest among persons aged 50-59 years. Persons with ALS identified in the national databases tended to be older than those identified through the web portal. Males had a higher overall prevalence rate (4.8) than females (3.0) (Figure 3) across each data source individually and overall. The ratio of males to females was 1.56. The prevalence rate for whites (4.2) was twice that for blacks (2.0) (Figure 4).
Limitations
The findings in this report are subject to at least four limitations. First, ALS is not a notifiable disease in the United States (except for Massachusetts), and all newly diagnosed and prevalent ALS cases in the United States probably are not captured in the registry; therefore, the possibility of underascertainment exists. Second, data entry errors could have prevented records from merging correctly. However quality checks by individual variables indicate that the overall conclusions probably are unaffected because data entry errors are unlikely to have occurred in any substantial number of records. Third, the calculation of national ALS incidence is not possible at this time because the date of diagnosis is not captured through the large administrative databases. Finally, the registry has been officially active since October 2009 and is still adding more participants; therefore, it might not be representative of all persons with ALS.
Public Health Implications
ALS is a progressive and fatal neuromuscular disease for which no cure has been identified. Data collected by the registry are being used to better describe the incidence and prevalence of ALS in the United States and to help facilitate research. The combined approach of using national administrative databases and a self-enrollment web portal to collect data is novel and potentially could be used for other non-notifiable diseases (e.g., Parkinson's disease and multiple sclerosis) (2).
This is the first prevalence estimate of ALS for the United States that is based on national data. The establishment of the registry will allow for determining trends in prevalence of this disease as well as continuing research on risk factors. Surveillance is important to monitor changes in incidence and prevalence of a condition. Surveillance data also can be used in planning for health-care needs, detecting changes in health practices, and assessing the burden of disease.
To capture all cases of ALS, ATSDR is working with ALS advocacy and support groups, researchers, health-care professionals, and others to promote the registry. To further enhance and strengthen the registry, ATSDR is 1) adding new modules to the portal as necessary to examine other potential risk factors, 2) conducting a feasibility study (concluding Fall 2015) for a novel ALS biorepository linked to the registry that would potentially provide biologic specimens from patient enrollees to help researchers learn more about disease etiology, 3) engaging in surveillance activities in selected states and large metropolitan areas to test the completeness of the registry and calculate incidence in these areas, and 4) using the registry to recruit patient enrollees for new clinical trials and epidemiologic studies. Identifying possible risk factors for ALS through close partnerships with academic researchers and enrolling persons with ALS in the registry will facilitate further exploration of the etiology of ALS.
Reference
- US Public Health Service. ALS Registry Act. Washington, DC: 110th Congress. Public Law 2008;122 Stat 4047:110-373. Available at http://wwwn.cdc.gov/als/Download/ALS%20Registry%20Act%20(Public%20Law%20110-373).pdf.
- Horton DK, Mehta P, Antao V. Quantifying a nonnotifiable disease in the United States: the National Amyotrophic Lateral Sclerosis Registry model. JAMA 2014;312:1097-8.
FIGURE 1. National Amyotrophic Lateral Sclerosis (ALS) Registry methodology
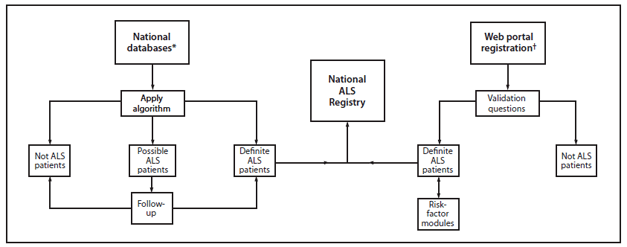
* Maintained by Medicare, Medicaid, the Veterans Health Administration, and the Veterans Benefits Administration.
† Available at http://www.cdc.gov/als.
Alternate Text: The figure is a flow chart that presents the methodology used by the National Amyotrophic Lateral Schlerosis Registry to determine cases of ALS.
FIGURE 2. Prevalence rates* for cases of amyotrophic lateral sclerosis, by age group — National Amyotrophic Lateral Sclerosis Registry, United States, October 19, 2010-December 31, 2011
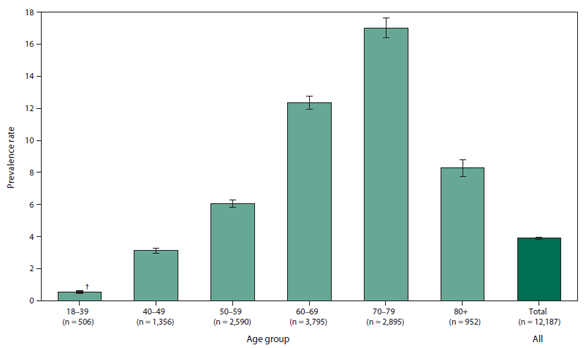
* Per 100,000 population.
† 95% confidence interval.
Alternate Text: The figure is a bar chart that presents the rates of cases of amyotrophic lateral schlerosis by age group for persons aged ≥18 years during October 19, 2010 to December 31, 2011. Rates were highest for persona aged 70-79 years and lowest for those aged 18-39.
FIGURE 3. Prevalence rates* for cases of amyotrophic lateral sclerosis, by sex — National Amyotrophic Lateral Sclerosis Registry, United States, October 19, 2010-December 31, 2011
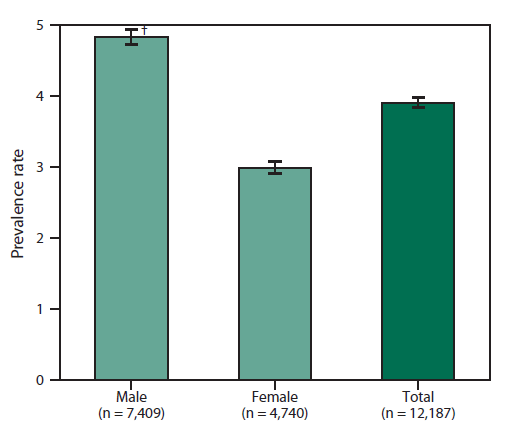
* Per 100,000 population.
† 95% confidence interval.
Alternate Text: The figure is a bar chart that presents the rates of cases of amyotrophic lateral schlerosis by sex for persons aged ≥18 years during October 19, 2010 to December 31, 2011. Rates were higher for males than females.
FIGURE 4. Prevalence rates* for cases of amyotrophic lateral sclerosis, by race — National Amyotrophic Lateral Sclerosis Registry, United States, October 19, 2010-December 31, 2011
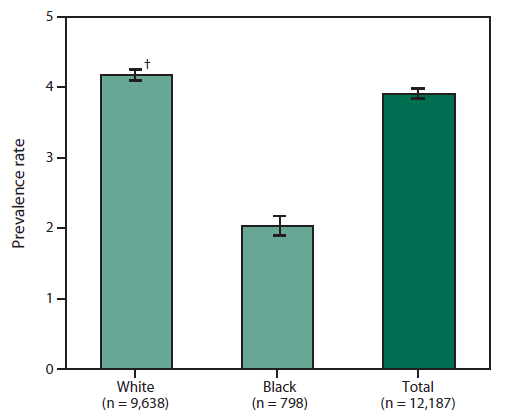
* Per 100,000 population.
† 95% confidence interval.
Alternate Text: The figure is a bar chart that presents the rates of cases of amyotrophic lateral schlerosis by age race for persons aged ≥18 years during October 19, 2010 to December 31, 2011. Rates were nearly double for whites than blacks.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


