Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Raccoon Roundworms in Pet Kinkajous --- Three States, 1999 and 2010
Baylisascaris procyonis (BP) is the common roundworm of raccoons (Procyon lotor). Adult BP live in the small intestine of this host, where they produce eggs that are passed in the feces. BP eggs ingested by nondefinitive host species hatch in the intestine, producing larvae that can migrate widely, causing visceral, ocular, or neural larva migrans (1). Cases of neural larva migrans in humans caused by BP likely acquired from raccoons have resulted in severe encephalitis with permanent deficits and in death (1--3). Although raccoons are the most common definitive host of BP in North America, some other carnivores, including domestic dogs, can serve as definitive hosts, making them a potential source of human disease (1). Less well-documented is infection in procyonids other than raccoons (e.g., kinkajous [Potos flavus] [Figure 1], coatis [Nasua spp.], olingos [Bassaricyon spp.], and ringtails [Bassariscus astutus]) and the potential for transmission from these species to humans. This report describes cases of BP infection in pet kinkajous that placed humans at risk for infection. Avoiding contact with feces from potentially infected animals and routine deworming of pets, including dogs and exotic species that might host this parasite, will prevent infection with BP.
Tennessee
In early April 2010, a pet kinkajou aged 10 weeks was seen by a veterinarian in rural, northeast Tennessee for a routine health examination. The fecal examination revealed numerous ascarid eggs tentatively identified as BP and later confirmed as BP by a commercial laboratory. The veterinarian treated the kinkajou with albendazole, discussed risks and precautionary measures with the owner, and alerted the Tennessee Department of Health (TDH) to the potential human exposures.
The kinkajou had been purchased at a storefront exotic pet shop in eastern Tennessee on March 18, 2010. A TDH state public health veterinarian encouraged the store owners to consult with their veterinarian and treat the remaining kinkajous in the store. The kinkajou in question had been imported to Tennessee from a south Florida facility. Personnel from TDH and the Animal Care division of the U.S. Department of Agriculture's Animal and Plant Health Inspection Service (USDA-AC) jointly visited the store to obtain information on recent purchases and to pursue testing of the other exotic animals.
The USDA-AC inspector collected acquisition and disposition records for 13 kinkajous that had been available for sale by the pet store during the preceding 2 years. Twelve kinkajous had been purchased by the store, and one was the offspring of the pet store's resident breeding pair. Eleven of 13 had been sold, and two were awaiting sale. TDH collected fecal samples from the cage floors of various exotic mammals and birds. Recommendations were made to the store owner concerning BP, including regular fecal cleanup and disposal, decontamination of cages and enclosures, and regular fecal examination and deworming of the animals. Although kinkajou fecal samples were not available at the time of the store visit, on April 12 and 13, samples were obtained from a littermate of the index positive animal and a breeding female that was a pet of the store owners. No parasites were detected in either animal.
The family that purchased the kinkajou included children aged 2 and 5 years. They had minimal contact with the animal and its cage and were judged to have a low risk for infection. Additionally, the family reported good hand hygiene practices. Papers lining the cage were changed on a daily basis, and feces outside the cage were disposed of immediately. As an additional precaution, the family physician prescribed treatment of family members with a single dose of mebendazole, and later prescribed for each a 10-day course of albendazole. The family remained free of clinical illness. During the pet store investigation, regional epidemiologists learned that animal care duties were shared by the two store owners and their two children aged 8 and 12 years. None reported illness, and none were treated.
A Tennessee public health veterinarian contacted the five residents who had purchased kinkajous from the pet store during the preceding year to notify them of the positive case and to offer information on free testing (conducted by Purdue University [West Lafayette, Indiana]), treatment, and cleaning. The remaining six kinkajous were purchased by residents of Michigan, Kentucky, North Carolina, and Florida; this information was shared with the respective state veterinary public health officials. The offer of free testing was extended to the two other Tennessee breeders from whom the pet store had purchased kinkajous during the preceding year. One of these submitted samples from three additional kinkajous (the breeding pair and one offspring). No parasite eggs were detected in pooled fecal samples.
Florida
The positive juvenile kinkajou in Tennessee had been purchased from a breeder in Miami-Dade County. Notification from TDH to the Florida Department of Health (FDOH) prompted a joint site visit by USDA-AC and Miami-Dade County Health Department (MDCHD) personnel to the breeder's facility on April 12, 2010. The purpose of the visit was to conduct employee interviews using a risk assessment questionnaire, assess sanitary conditions, monitor kinkajou handling, and learn about the facility's deworming practices.
The breeding operation was fenced and maintained by the owner and one employee. The breeder raised exotic birds and kinkajous (a total of 44 kinkajous in 21 enclosures); the kinkajous were obtained from Guyana (on the northern coast of South America), with no new additions since 2008. The kinkajous were kept in their own compound in raised wire cages with nest boxes; their feces fell directly to unprotected ground below. The housing area was cleaned daily, and animals received regular veterinary care, including fecal examinations and treatment of parasitic infections, general biannual deworming, and preshipment deworming of any purchased offspring. According to the breeder's veterinarian, no BP infections had ever been identified.
Fecal samples from animals in each of the 21 enclosures were examined, and no BP eggs were found. During a subsequent USDA-AC visit, soil samples were collected from under the breeding cages of the two juveniles sent to Tennessee. Samples (weighing 40--50 g) from each of the two bags of soil were processed and examined for eggs. Infective (larvated) BP eggs (Figure 2) were found in both samples, with one being heavily contaminated.
Both the owner and employee reported regularly using good hand hygiene while working with the animals. Neither reported any illness, and they were not treated.
Two other kinkajous had been sold by the Florida breeder to the Tennessee pet store and then purchased by a wildlife rehabilitation facility in Hernando County, Florida. These kinkajous were part of a public exhibit and were housed in an area accessible to domestic animals and wildlife. Hernando County Health Department officials made a site visit with USDA-AC personnel and sent the facility owner an informational letter describing infection control and prevention measures. The owner provided fecal samples from the facility's kinkajous, which tested negative for BP. All animal and environmental samples from Florida were tested at Purdue University.
Indiana
In an unrelated event in January 1999, a veterinarian in southern Indiana examined two pet kinkajous acquired 5 weeks earlier that were ill and subsequently died from bacterial infections. They were from a small, private, exotic animal collection that included kinkajous, coatis, spider monkeys, and various other species. The two kinkajous were necropsied and found to have approximately 20--25 BP adults in their small intestine. One animal had passed an ascarid in its feces shortly after acquisition. Based on the developmental period of the parasite (approximately 2.0--2.5 months)(1), the BP infection was present before the owner took possession of the animals. The veterinarian reported that the premises were clean and the animals well cared for, including regular feces removal and disposal. Recommendations were made for deworming existing animals, increased sanitation, and environmental decontamination of the kinkajou and coati enclosures. No associated human illnesses were reported.
Reported by
KR Kazacos, DVM, Purdue Univ, West Lafayette; TP Kilbane, DVM, Evansville, Indiana. KD Zimmerman, DVM, Bluff City; T Chavez-Lindell, MPH, B Parman, T Lane, LR Carpenter, DVM, AL Green, DVM, Tennessee Dept of Health. PM Mann, MPH, TW Murphy, Miami-Dade County Health Dept, Miami; B Bertucci, AC Gray, MPH, Hernando County Health Dept, Brooksville; TL Goldsmith, DVM, Coconut Grove; M Cunningham, DVM, Florida Fish and Wildlife Conservation Commission; DR Stanek, DVM, C Blackmore, DVM, Florida Dept of Health. MJ Yabsley, PhD, Univ of Georgia. SP Montgomery, DVM, E Bosserman, MPH, Div of Parasitic Diseases and Malaria, Center for Global Health, CDC.
Editorial Note
This report describes the potential for human exposure to the raccoon roundworm from pet kinkajous. As part of the exotic pet trade, kinkajous are imported from South America and bred in captivity; the offspring are sold as exotic pets. In addition to raccoons, adult BP have been described in a kinkajou from Colombia and in another procyonid, the bushy-tailed olingo (Bassaricyon gabbii) (4).
Based on raccoon studies, infected animals can shed thousands to millions of BP eggs in their feces daily (1). These eggs become infective in a minimum of 11--14 days under optimal conditions and, given adequate moisture, can remain infective for years (1). After ingesting infective eggs from fecal-contaminated articles or environments, larvae hatch in the intestinal tract and undergo a somatic migration, entering various organs and tissues. Although the incubation period in humans is undefined, severe neurologic disease can develop as soon as 2--4 weeks after ingestions of infective eggs. Severe disease occurs when larvae enter the brain or eyes causing neurologic or ocular manifestations (1--3). Although a single larva in the eye can result in ocular problems, the severity of neurologic disease is dependent upon the number of larvae migrating to the brain. Fatal human cases have been documented in children who had ingested very large numbers of eggs (5).
Prevention of disease is based on aborting migration of BP larvae to organs and tissues by postexposure treatment. The sensitivity of diagnostic tests, including tests for antibody to BP, are limited during the period after infection and before clinical signs develop, and treatment decisions must be based on risk assessment. If likelihood of infection is high, treatment with albendazole at 25--50 mg/kg per day by mouth for 10--20 days might prevent disease. However, the efficacy of postexposure treatment has not been studied extensively. The likelihood of infection might depend on factors such as known oral exposure to raccoon or other parasite host feces, presence of Baylisascaris eggs in the feces of the implicated animal or animals, and suspected oral exposure to raccoon feces in an area where the prevalence of raccoon infection is known to be high. Timing of postexposure treatment also is important because BP larvae can cause neurologic disease as soon as 2 weeks after ingestion of infective eggs; treatment should be initiated as soon as possible, ideally within 3 days.
The results of this investigation extend the list of potential sources of BP egg contamination to include pet kinkajous. Considering the difficulty of diagnosing and treating BP infection in humans, prevention of infection is of paramount importance. Pet dogs, kinkajous and other procyonids, and skunks (host of a related Baylisascaris species) (1), which might be kept as exotic pets, should receive routine veterinary care, including regular dewormings and periodic fecal examinations. Feces from potentially infected animals should be carefully disposed of at least weekly, and contaminated areas should be treated with steam or scalding/boiling water to kill residual eggs (1,3,6). Additional information about raccoon roundworm is available at http://www.cdc.gov/parasites/baylisascaris.
Acknowledgments
The findings in this report are based, in part, on contributions by G Gaj, DVM, RA Willems, DVM, ME Moore, DVM, Animal Care; MP Milleson, Wildlife Svcs, Animal and Plant Health Inspection Svc, US Dept of Agriculture.
References
- Kazacos KR. Baylisascaris procyonis and related species. In: Samuel WM, Pybus MJ, Kocan AA, eds. Parasitic diseases of wild mammals, 2nd ed. Ames, Iowa: Iowa State University Press; 2001:301--41.
- Murray WJ, Kazacos KR. Raccoon roundworm encephalitis. Clin Infect Dis 2004;39:1484--92.
- Gavin PJ, Kazacos KR, Shulman ST. Baylisascariasis. Clin Microbiol Rev 2005;18:703--18.
- Overstreet RM. Baylisascaris procyonis (Stefanski and Zarnowski, 1951) from the kinkajou, Potos flavus, in Colombia. Proc Helminthol Soc Wash 1970;37:192--5.
- Fox AS, Kazacos KR, Gould NS, et al. Fatal eosinophilic meningoencephalitis and visceral larva migrans caused by the raccoon ascarid Baylisascaris procyonis. N Engl J Med 1985;312:1619--23.
- Shafir SC, Wang W, Sorvillo FJ, et al. Thermal death point of Baylisascaris procyonis eggs. Emerg Infect Dis 2007;13:172--3.
What is already known on this topic?
Persons who accidentally ingest Baylisascaris procyonis (BP) eggs, which are shed in the feces of infected raccoons, can develop serious neurologic disease.
What is added by this report?
This report documents that kinkajous, exotic species often kept as pets, also can be infected with BP and can be a potential source of infection to persons in contact with these animals.
What are the implications for public health practice?
Increased awareness among pet owners, veterinarians, and health professionals will help reduce the risk for infection with BP by promotion of appropriate hygiene practices, including prompt removal of fecal material, and routine testing and deworming of potentially infected animals kept as pets.

Photo/Thinkstock
Alternate Text: The figure above shows a kinkajou (Potos flavus).
FIGURE 2. Infective (larvated) Baylisascaris procyonis egg uncovered from the soil under a kinkajou cage --- Florida, April 2010
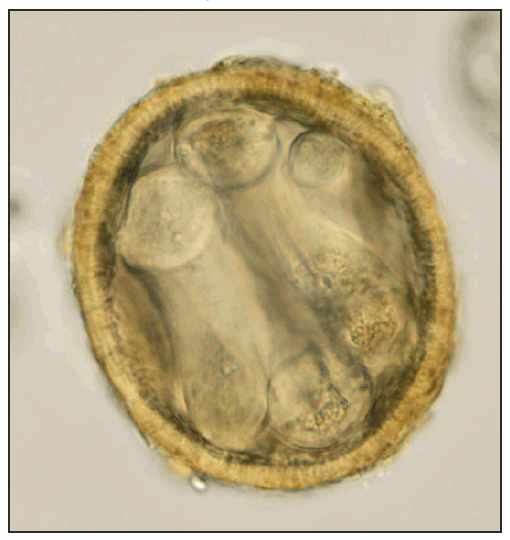
Photo/K. Kazacos
Alternate Text: The figure above shows an infective (larvated) Baylisascaris procyonis egg, uncovered from the soil under a kinkajou cage in Florida in April of 2010.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


