Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Balamuthia mandrillaris Transmitted Through Organ Transplantation --- Mississippi, 2009
On December 14, 2009, a physician in Mississippi contacted CDC to report possible transplant-transmitted encephalitis in two kidney transplant recipients who shared the same organ donor. Histopathologic testing of donor autopsy brain tissue at CDC showed amebae, and subsequent testing of specimens from the donor and the two kidney recipients confirmed transmission by transplantation of Balamuthia granulomatous amebic encephalitis (GAE), a rare disease caused by Balamuthia mandrillaris, a free-living ameba found in soil (1). One kidney recipient, a woman aged 31 years, died; the other recipient, a man aged 27 years, survived with neurologic sequelae. Recipients of the heart and liver from the same donor received preemptive therapy and have shown no signs of infection. The donor, a previously healthy boy aged 4 years, was presumed to have died from acute disseminated encephalomyelitis (ADEM), an autoimmune neurologic disease, after infection with influenza A. An investigation was conducted by the state health departments in Mississippi, Kentucky, Florida, and Alabama and CDC to characterize the cases, elucidate possible exposures in the donor, and develop recommendations for early detection and prevention. This is the first reported transmission of Balamuthia by organ transplantation. Clinicians should be aware of Balamuthia infection as a potentially fatal cause of encephalitis. Organ procurement organizations (OPOs) and transplant centers should be aware of the potential for Balamuthia infection in donors with encephalitis of uncertain etiology, and OPOs should communicate this elevated risk for infection to transplant centers so they can make an informed risk assessment in the decision to accept an organ.
Organ Donor
The organ donor, a boy aged 4 years from Kentucky, was living with relatives in Mississippi in October 2009, when he developed a transient febrile illness. He was diagnosed with influenza A infection by rapid influenza test on October 25 and prescribed antivirals; his symptoms resolved without hospitalization. On November 3, the boy had sudden onset of headache and seizures and was hospitalized (Table 1). Cerebrospinal fluid (CSF) demonstrated lymphocytic pleocytosis (170 white blood cells/mm3) and normal protein (29 mg/dL); magnetic resonance imaging (MRI) of the brain showed numerous small enhancing lesions and edema (Table 2). An extensive search for viral, bacterial, and fungal etiologies of encephalitis was unrevealing. His clinical presentation, CSF findings, and MRI were thought to be most consistent with a diagnosis of ADEM, an immune-mediated encephalitis that can follow influenza or other infections. He was treated symptomatically and discharged on November 6.
The boy was readmitted on November 10 with recurrent seizures. MRI of the brain demonstrated progression of several of the enhancing lesions; CSF again demonstrated lymphocytic pleocytosis (150 cells/mm3) and normal protein (44 mg/dL) (Table 2). He was treated for presumed worsening ADEM with intravenous corticosteroids and immunoglobulin. He developed subarachnoid hemorrhage and brain stem herniation on November 18 and was pronounced brain dead the next day. His heart, liver, and kidneys were transplanted into four recipients at three different transplant centers on November 20. On December 16, histopathologic examination of the donor's brain tissue at CDC revealed the presence of abundant amebae morphologically suggestive of Balamuthia (Figure); empiric treatment for both kidney recipients was initiated later that day, in consultation with CDC. On December 17, immunohistochemical and indirect immunofluorescent stains (Figure) revealed antigens of free-living amebae in the donor's brain tissue; polymerase chain reaction (PCR) results confirmed Balamuthia infection.
Kidney Recipient A
Kidney recipient A, a woman aged 31 years, underwent transplantation for end-stage renal disease resulting from hypertension and diabetes. On December 10, post-transplant day (PTD) 20, she reported onset of right leg twitching and neck spasms, numbness, headache, nausea, and seeing flashing lights (Table 1). She was evaluated in an emergency department, where she was treated with benzodiazepines and discharged with muscle relaxants; no neuroimaging or lumbar puncture was performed. On December 12, she was found unresponsive at home and taken back to the emergency department, where she had a generalized seizure and was admitted; the next day, she was transferred to the intensive-care unit. MRI of the brain demonstrated numerous ring-enhancing lesions. CSF initially showed a normal white blood cell count (3 cells/mm3) and elevated protein (75 mg/dL); however, another specimen collected on December 15 revealed a neutrophilic pleocytosis (507 cells/mm3) and increased protein (142 mg/dL) (Table 2). On December 16, she underwent brain biopsy. On December 18, histopathologic examination of the brain tissue at CDC revealed amebae; immunohistochemical stains detected antigens of free-living amebae, and PCR confirmed Balamuthia infection. She was treated with pentamidine, sulfadiazine, flucytosine, fluconazole, and azithromycin. Miltefosine, an antileishmanial and antineoplastic agent, was added on December 25 under an emergency investigational new drug (IND) protocol. Despite several weeks of intensive care, she deteriorated neurologically and died on February 3 (PTD 75).
Kidney Recipient B
Kidney recipient B, a man aged 27 years, underwent transplantation for end-stage renal disease resulting from focal segmental glomerulosclerosis. On December 10 (PTD 20), he had sudden onset of severe headache and vomiting and was examined at a local emergency department early the next morning, where he was diagnosed with sinusitis and discharged on amoxicillin-clavulanic acid (Table 1). Later that day, he developed altered mental status and seizures and was admitted to a regional hospital. A lumbar puncture was performed; CSF demonstrated 1 white blood cell/mm3 and slightly elevated protein (69 mg/dL) (Table 2). On December 13, he was transferred to the intensive-care unit at the same hospital as kidney recipient A. CSF that day revealed mild pleocytosis (19 cells/mm3) and slightly increased protein (74 mg/dL). MRI of the brain showed numerous ring-enhancing lesions. The man was treated with the same combination of drugs as kidney recipient A, including miltefosine obtained under IND. Balamuthia infection was confirmed by PCR and culture on a CSF specimen drawn December 29. After 2 months in a coma, the man had a slow but significant recovery of cognitive and motor function and was discharged to a rehabilitation facility on April 28 (PTD 159). He was discharged home June 11. His neurologic sequelae included residual right arm paralysis, bilateral leg weakness, and intermittent vision loss; however, he performed most activities of daily living independently.
Heart Recipient
The heart recipient, a boy aged 2 years, underwent transplantation for restrictive cardiomyopathy. When the kidney recipients were diagnosed with Balamuthia GAE, the boy was asymptomatic. On December 17 (PTD 27), he was hospitalized for evaluation. MRI of the brain was normal, and testing of CSF, serum, and endomyocardial tissue at CDC showed no evidence of Balamuthia infection. The boy was treated for presumed Balamuthia exposure with a 6-week course of intravenous pentamidine, azithromycin, and fluconazole, followed by 5 weeks of oral azithromycin. He remains well.
Liver Recipient
The liver recipient, a boy aged 7 years, underwent transplantation for end-stage liver disease resulting from alpha-1-antitrypsin deficiency. The boy was asymptomatic when the kidney recipients were diagnosed with Balamuthia GAE, and he was hospitalized for evaluation on December 17. MRI of the boy's brain was normal, and testing of CSF, serum, and liver tissue at CDC showed no evidence of Balamuthia infection. He was treated for presumed Balamuthia exposure with a 1-month course of intravenous pentamidine, fluconazole, azithromycin, and sulfadiazine. He remains well.
Public Health Investigation
Interviews with the donor's family revealed that he had lived in Kentucky, Florida, and Mississippi during the 2 years before his death. He frequently played outdoors and had soil exposure in all of these locations. He occasionally played in a wading pool; the water supply for drinking and recreation in Florida was untreated well water. No environmental sampling was performed because Balamuthia is thought to be ubiquitous in the environment.
Approximately 4 months before his first seizure, the boy had become more irritable and emotionally labile. His family also noted regression of toilet training and an infrequent, sporadic tremor of the right hand that began at about the same time. He had no history of immunocompromising conditions. No medical evaluation of family members was conducted.
Reported by
S Schlessinger, MD, Mississippi Organ Recovery Agency, Flowood; K Kokko, MD, J Fratkin, MD, F Butt, MD, A Hawxby, MD, M Todaro, PharmD, H Henderson, MD, A Seawright, DNP, C Parker, MD, Univ of Mississippi Medical Center; P Byers, MD, Mississippi Dept of Health. R Gonzalez-Peralta, MD, L Kayler, MD, L Fauerbach, R Lawrence, MD, A Haafiz, MD, Shands Hospital, Univ of Florida; D Stanek, DVM, R Hammond, Florida Dept of Health. D Thoroughman, PhD, Kentucky Dept for Public Health. T Pippen, S Johnson, Alabama Dept of Public Health. W Mahle, MD, G Lyon III, MD, K Laporte, K Kanter, MD, Children's Healthcare of Atlanta, Emory Univ, Atlanta; M Ivey, MPH, K Arnold, MD, S Lance, DVM, Georgia Div of Public Health. E Navarro-Almario, Food and Drug Admin. E Farnon, MD, M Kuehnert, MD, Div of Healthcare Quality Promotion; W Shieh, MD, C Paddock, MD, S Zaki, MD, C Drew, DVM, A Schmitz, DVM, J Sejvar, MD, Div of High-Consequence Pathogens and Pathology; R Sriram, PhD, G Visvesvara, PhD, M Beach, PhD, J Yoder, MPH, S Roy, MD, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases; Y Qvarnstrom, PhD, R Bandea, PhD, A daSilva, PhD, E Bosserman, MPH, Div of Parasitic Diseases and Malaria, Center for Global Health; P Budge, MD, E Lutterloh, MD, EIS officers, CDC.
Editorial Note
This report is the first to describe transmission of Balamuthia through organ transplantation. However, a second cluster of patients with transplant-transmitted Balamuthia was confirmed at CDC on August 27, 2010 (2). Balamuthia infection is extremely rare, with fewer than 200 human cases recognized worldwide since Balamuthia was found to be a human pathogen in 1990 (3,4). The true magnitude of disease caused by Balamuthia is unknown because Balamuthia GAE often is misdiagnosed as other neurologic diseases (1,3). Once infection progresses to encephalitis, it is almost always fatal. Infection occurs in both immunocompromised and otherwise healthy persons, and often in children, although cases have occurred in patients across the age spectrum (5). Because of the rarity of Balamuthia GAE, risk factors are poorly defined, but might include exposure to soil or stagnant water, young age, and Hispanic ethnicity (3).
Balamuthia has been isolated from soil and dust and is thought to be present worldwide (6). Routes of infection might include exposure of mucous membranes or nonintact skin to cysts or trophozoites in soil. Balamuthia has not been isolated from water, but water also might serve as a vehicle for infection (1). Cutaneous lesions have preceded Balamuthia GAE in some cases, primarily those reported in South America (7). These lesions often are on the central face, suggesting nasal exposure; but they also have been reported on the extremities. Extension to the brain might occur through hematogenous spread or by direct extension through the nasal cavity or sinuses (1). Why some patients develop cutaneous lesions before onset of neurologic disease and others do not is unknown. In a series of 10 Balamuthia cases in California, common signs and symptoms of Balamuthia GAE were headache, altered mental status, and cranial nerve abnormalities (3). Although the incubation period for Balamuthia GAE has been postulated as ranging from weeks to 2 years, the two kidney recipients in this report had onset of symptoms only 20 days after transplantation.
Successful treatment of Balamuthia GAE has been reported in some, but not all, patients administered a combination of flucytosine, pentamidine, sulfadiazine, fluconazole or amphotericin B, azithromycin or clarithromycin, and miltefosine (3,8). However, optimal therapy has not been determined. Optimal preemptive therapy for asymptomatic recipients after transplant of an infected organ also is unknown. Miltefosine is active against Balamuthia in vitro and was recently used with success in combination therapy for Balamuthia GAE in Peru (9). Miltefosine is not marketed in the United States but can be available through single patient IND.*
Balamuthia is one of several agents of severe or fatal encephalitis (e.g., West Nile virus, lymphocytic choriomeningitis virus, and rabies virus) that have been transmitted through organ transplantation in recent years (10). Organ donors are screened to identify infectious risks in accordance with policies set by the Organ Procurement and Transplantation Network,† which is overseen by the United Network for Organ Sharing through a contract with the Health Resources and Services Administration. However, the number of pathogens screened is limited and creating standards that eliminate all risk for infectious disease transmission is not feasible. Therefore, physicians and organ procurement organizations should be aware of the possibility of transmitting Balamuthia and other potentially fatal infections from donors with encephalitis of uncertain etiology, even after testing for usual agents of encephalitis has shown negative results (10). Balamuthia infection should be considered in patients who might have an infectious encephalitis, particularly those with elevated CSF protein, CSF pleocytosis (white blood cells >5/mm3), and enhancing lesions on MRI (3).
Clinicians should be aware of Balamuthia as a cause of skin lesions and encephalitis and should report all suspected cases of transplant-transmitted infection to public health departments and organ procurement organizations to enable prompt evaluation and treatment of all recipients from an infected donor. OPOs and transplant centers should be aware of the potential for Balamuthia infection in donors with encephalitis of uncertain etiology, and OPOs should communicate this elevated risk for infection to transplant centers so they can make an informed risk assessment in the decision to accept an organ.§
References
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 2007;50:1--26.
- CDC. Transplant-transmitted Balamuthia mandrillaris---Arizona, 2010. MMWR 2010;59:1182.
- CDC. Balamuthia amebic encephalitis---California, 1999--2007. MMWR 2008;57:768--71.
- Visvesvara GS, Martinez AJ, Schuster FL, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 1990;28:2750--6.
- Cary LC, Maul E, Potter C, et al. Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics 2010;125:e699--703.
- Niyyati M, Lorenzo-Morales J, Rezaeian M, et al. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 2009;106:279--81.
- Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clin 2003;21:655--68, viii.
- Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med 2004;128:466--8.
- Martinez DY, Seas C, Bravo F, et al. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 2010;51:e7--11.
- Kotton C. Zoonoses in solid-organ and hematopoietic stem cell transplant recipients. Clin Inf Dis 2007;44:857--66.
* For information regarding a single patient IND for miltefosine, contact the Food and Drug Administration's Division of Special Pathogen and Transplant Products at 301-796-1600 (1-888-INFO-FDA after hours).
† Additional information available at http://optn.transplant.hrsa.gov/policiesandbylaws/policies.asp.
§ Additional information available at http://www.cdc.gov/balamuthia.
What is already known on this topic?
Balamuthia madrillaris is a free-living ameba found in soil worldwide that causes skin lesions and Balamuthia granulomatous amebic encephalitis (GAE), a rare central nervous system infection that usually is fatal; because of its rarity, Balamuthia GAE is likely to be misdiagnosed as another neurologic disease.
What is added by this report?
This is the first report of Balamuthia madrillaris transmission through organ transplantation; two of four recipients from an organ donor thought to have a noninfectious encephalitis developed GAE 20 days after transplantation, one of whom died.
What are the implications for public health practice?
Organ procurement organizations (OPOs) and transplant centers should be aware of the potential for Balamuthia infection in donors with encephalitis of uncertain etiology, and OPOs should communicate this elevated risk for infection to transplant centers so they can make an informed risk assessment in the decision to accept an organ.
FIGURE. Organ donor brain tissue revealing amebae suggestive of Balamuthia (indicated by arrows) (A), and immunohistochemical staining showing antigens (red) of free-living amebae (B)*
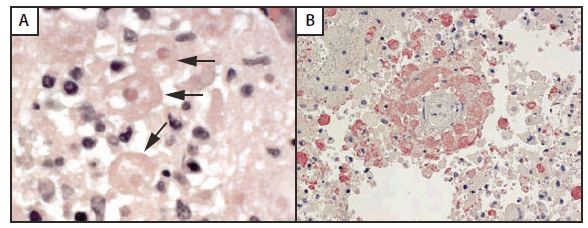
* Original magnifications: 158x (A), 100x (B).
Photomicrographs/CDC
Alternate Text: The figure above shows organ donor brain tissue revealing amebae suggestive of Balamuthia (indicated by arrows) (A), and immunohistochemical staining showing antigens (red) of free-living amebae (B). On Dec. 17, 2009, immunohistochemical and indirect immunofluorescent stains revealed antigens of free-living amebae in the brain tissue; polymerase chain reaction results confirmed Balamuthia infection.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


