Vagus nerve stimulation
Vagus nerve stimulation (VNS) is a medical treatment that involves delivering electrical impulses to the vagus nerve. It is used as an add-on treatment for certain types of intractable epilepsy and treatment-resistant depression. Frequent side effects include coughing and shortness of breath.[1] Serious side effects may include trouble talking and cardiac arrest.[1]
| Vagus nerve stimulation | |
|---|---|
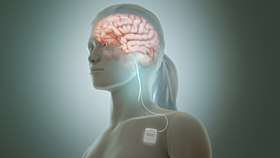 Electrical stimulation of vagus nerve. | |
| Other names | Vagal nerve stimulation |
Medical use
VNS devices are used to treat drug-resistant epilepsy and treatment-resistant major depressive disorder (TR-MDD).[2][3][4] Specifically, it is used for treatment-resistant focal epilepsy.[3] As of 2017 the efficacy of VNS for TR-MDD was unclear.[2][4]
Adverse events
For the treatment of epilepsy generally the left vagus nerve is stimulated at mid-cervical region. The adverse effects of this stimulation include cardiac arrest,[5] bradycardia,[6] voice alteration and hoarseness, cough, shortness of breath, pain, a tingling sensation, nausea, and headache;[3] difficulty swallowing has also been reported as common,[7] as well as sleepiness.
In randomized controlled trials for epilepsy conducted in the United States, one-third of the subjects had some type of an increase in seizures, with 17 percent having greater than a 25 percent increase, some had 100 percent increase or more.[8]
Mechanism of action
As of 2017 little was understood about exactly how vagal nerve stimulation modulates mood and seizure control.[2]
The vagus is the tenth cranial nerve and arises from the medulla; it carries both afferent and efferent fibers. The afferent vagal fibers connect to the nucleus of the solitary tract which in turn projects connections to other locations in the central nervous system.[3] Proposed mechanisms include an anti-inflammatory effect,[3] as well as changes in monoamines.
Devices and procedures
The device consists of a generator the size of a matchbox that is implanted under the skin below the person’s collarbone. Lead wires from the generator are tunnelled up to the patient’s neck and wrapped around the left vagus nerve at the carotid sheath, where it delivers electrical impulses to the nerve.[2]
Implantation of the VNS device is usually done as an out-patient procedure. The procedure goes as follows: an incision is made in the upper left chest and the generator is implanted into a little "pouch" on the left chest under the collarbone. A second incision is made in the neck, so that the surgeon can access the vagus nerve. The surgeon then wraps the leads around the left branch of the vagus nerve, and connects the electrodes to the generator. Once successfully implanted, the generator sends electric impulses to the vagus nerve at regular intervals. The left vagus nerve is stimulated rather than the right because the right plays a role in cardiac function such that stimulating it could have negative cardiac effects.[4][9] The "dose" administered by the device then needs to be set, which is done via a magnetic wand; the parameters adjusted include current, frequency, pulse width, and duty cycle.[4]
"Wearable" devices are being tested and developed that involve transcutaneous stimulation and do not require surgery. Electrical impulses are targeted at the aurical (ear), at points where branches of the vagus nerve have cutaneous representation; such devices had been tested in clinical trials for treatment resistant major depressive disorder as of 2017.[4][10]
History
In 1997, the US Food and Drug Administration's neurological devices panel met to consider approval of an implanted vagus nerve stimulator (VNS) for epilepsy, requested by Cyberonics (which was subsequently renamed to LivaNova).[2]
The FDA approved an implanted VNS for TR-MDD in 2005.[4]
In April 2017, the FDA cleared marketing of a handheld noninvasive vagus nerve stimulator, called "gammaCore" and made by ElectroCore LLC, for episodic cluster headaches, under the de novo pathway.[11][12] In January 2018, the FDA cleared a new user for that device for the treatment of migraine pain in adults under a 510(k) based on the de novo clearance.[13][14]
Controversy
Although the use of VNS for TRD has been endorsed by the American Psychiatric Association, the FDA's approval of VNS for TRD remains controversial. According to Dr. A. John Rush, vice chairman for research in the Department of Psychiatry at the University of Texas Southwestern Medical Center at Dallas, results of the VNS pilot study showed that 40 percent of the treated patients displayed at least a 50 percent or greater improvement in their condition, according to the Hamilton Depression Rating Scale.[15][16] Many other studies concur that VNS is indeed efficacious in treating depression. However, these findings do not take into account improvements over time in patients without the device. In the only randomized controlled trial VNS failed to perform any better when turned on than in otherwise similar implanted patients whose device was not turned on.[17]
Research
Because the vagus nerve is associated with many different functions and brain regions, clinical research has been done to determine its usefulness in treating other illnesses, including various anxiety disorders,[18] obesity,[19][20] alcohol addiction,[21] chronic heart failure,[22] prevention of arrhythmias that can cause sudden cardiac death,[23] autoimmune disorders,[24][25] and several chronic pain conditions.[26]
VNS has also been studied in small trials of people with neurodevelopmental disorders, generally who also have had epilepsy, including Landau-Kleffner syndrome, Rett syndrome, and autism spectrum disorders.[27] VNS is being studied as of 2018 as a treatment for migraines and fibromyalgia.[28][29]
Transcutaneous
As of 2015 VNS devices were being developed that were not implanted, but rather transmitted signals through the skin, known as transcutaneous vagus nerve stimulation (tVNS). Electrical impulses are targeted at the auricle of the ear at points where branches of the vagus nerve are close to the surface.[4][10] It is non-invasive and based on the rationale that there is vagus nerve distribution on the surface of the ear. tVNS is being studied for stroke and the treatment of depression.[30][31]
See also
References
- "Vagus Nerve Stimulation". Cleveland Clinic. Retrieved 19 October 2018.
- Edwards, CA; Kouzani, A; Lee, KH; Ross, EK (September 2017). "Neurostimulation Devices for the Treatment of Neurologic Disorders". Mayo Clinic Proceedings. 92 (9): 1427–1444. doi:10.1016/j.mayocp.2017.05.005. PMID 28870357.

- Panebianco, M; Rigby, A; Weston, J; Marson, AG (3 April 2015). "Vagus nerve stimulation for partial seizures". The Cochrane Database of Systematic Reviews (4): CD002896. doi:10.1002/14651858.CD002896.pub2. PMID 25835947.

- Carreno, FR; Frazer, A (July 2017). "Vagal Nerve Stimulation for Treatment-Resistant Depression". Neurotherapeutics. 14 (3): 716–727. doi:10.1007/s13311-017-0537-8. PMC 5509631. PMID 28585221.
- Lenzer, Jeanne (2017) The Danger Within Us pg.109 ISBN 9780316343763
- Han p, frei mg, osorio i. Probable mechanisms of action of vagus nerve stimulation in humans with epilepsy: is a window into the brain [abstract]? epilepsia 1996;37 (5suppl):83s.
- Howland, RH (June 2014). "Vagus Nerve Stimulation". Current Behavioral Neuroscience Reports. 1 (2): 64–73. doi:10.1007/s40473-014-0010-5. PMC 4017164. PMID 24834378.
- "Neurological Devices Panel: Tenth Meeting transcript" (PDF). FDA. June 27, 1997. p. 125. Archived from the original (PDF) on August 19, 2000.
- Giordano, F; Zicca, A; Barba, C; Guerrini, R; Genitori, L (April 2017). "Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity". Epilepsia. 58 Suppl 1: 85–90. doi:10.1111/epi.13678. PMID 28386925.
- Leusden, J; Sellare, R; et al. (2015). "Transcutaneous Vagal Nerve Stimulation (tVNS): a new neuromodulation tool in healthy humans?". Frontiers in Psychology. 6 (102): 287–95. doi:10.3389/fpsyg.2015.00102. PMC 4322601. PMID 25713547.
- Brauser, Deborah (April 18, 2017). "FDA Approves Vagus Nerve Stimulation Device for Cluster Headache". Medscape.
- "GammaCore Device Classification under Section 513(f)(2)(de novo)". FDA. Retrieved 6 June 2018.
- Brauser, Deborah (January 29, 2018). "FDA Clears Vagus Nerve Stimulator for Migraine Pain". Medscape.
- "GammaCore 510(k) Premarket Notification". FDA. Retrieved 6 June 2018.
- Doctor's Guide: Vagus Nerve Stimulation Successful For Depression
- Neurology Channel: Vagus Nerve Stimulation Archived October 27, 2005, at the Wayback Machine
- FDA Summary of VNS Data
- Groves, Duncan A.; Brown, Verity J. (2005). "Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects". Neuroscience & Biobehavioral Reviews. 29 (3): 493–500. doi:10.1016/j.neubiorev.2005.01.004. PMID 15820552.
- de Lartigue, G (15 October 2016). "Role of the vagus nerve in the development and treatment of diet-induced obesity". The Journal of Physiology. 594 (20): 5791–5815. doi:10.1113/JP271538. PMC 5063945. PMID 26959077.
- Göbel, CH; Tronnier, VM; Münte, TF (30 June 2017). "Brain stimulation in obesity". International Journal of Obesity. 41 (12): 1721–1727. doi:10.1038/ijo.2017.150. PMID 28663570.
- Herremans SC, Baeken C (September 2012). "The current perspective of neuromodulation techniques in the treatment of alcohol addiction: a systematic review" (PDF). Psychiatria Danubina. 24 (Suppl 1): S14–20. PMID 22945180.
- Abraham WT, Smith SA (February 2013). "Devices in the management of advanced, chronic heart failure". Nature Reviews. Cardiology. 10 (2): 98–110. doi:10.1038/nrcardio.2012.178. PMC 3753073. PMID 23229137.
- Sabbah, HN (August 2011). "Electrical vagus nerve stimulation for the treatment of chronic heart failure". Cleveland Clinic Journal of Medicine. 78 Suppl 1: S24–9. doi:10.3949/ccjm.78.s1.04. PMC 3817894. PMID 21972326.
- Fox, Douglas (4 May 2017), Can Zapping the Vagus Nerve Jump-Start Immunity? : An experimental procedure is exposing links between nervous and immune systems, Scientific American
- Koopman, FA; van Maanen, MA; Vervoordeldonk, MJ; Tak, PP (2017). "Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis". J. Intern. Med. 282 (1): 64–75. doi:10.1111/joim.12626. PMID 28547815.
- Chakravarthy, K; Chaudhry, H; Williams, K; Christo, PJ (December 2015). "Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management". Current Pain and Headache Reports. 19 (12): 54. doi:10.1007/s11916-015-0528-6. PMID 26493698.
- Engineer, CT; Hays, SA; Kilgard, MP (2017). "Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders". Journal of Neurodevelopmental Disorders. 9: 20. doi:10.1186/s11689-017-9203-z. PMC 5496407. PMID 28690686.
- Johnson, RL; Wilson, CG (2018). "A review of vagus nerve stimulation as a therapeutic intervention". Journal of Inflammation Research. 11: 203–213. doi:10.2147/JIR.S163248. PMC 5961632. PMID 29844694.
- Puledda, F; Goadsby, PJ (April 2017). "An Update on Non-Pharmacological Neuromodulation for the Acute and Preventive Treatment of Migraine". Headache. 57 (4): 685–691. doi:10.1111/head.13069. PMID 28295242.
- Cai PY, Bodhit A, Derequito R, Ansari S, Abukhalil F, Thenkabail S, Ganji S, Saravanapavan P, Shekar CC, Bidari S, Waters MF, Hedna VS (June 2014). "Vagus nerve stimulation in ischemic stroke: old wine in a new bottle". Front. Neurol. 5: 107. doi:10.3389/fneur.2014.00107. PMC 4067569. PMID 25009531.
- Shiozawa, P; Silva, ME; Carvalho, TC; Cordeiro, Q; Brunoni, AR; Fregni, F (July 2014). "Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review". Arquivos de Neuro-Psiquiatria. 72 (7): 542–7. doi:10.1590/0004-282x20140061. PMID 25054988.
Further reading
- "Report casts doubt on VNS approval. - Free Online Library". www.thefreelibrary.com. Retrieved 2015-12-31.
- Feder, Barnaby J. (September 10, 2006). "Battle Lines in Treating Depression". The New York Times.