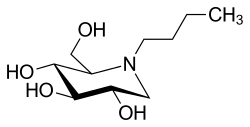
Miglustat
Miglustat (OGT 918, N-butyl-deoxynojirimycin) is a drug developed by Oxford GlycoSciences and marketed by Actelion and is used primarily to treat type I Gaucher disease (GD1). It is marketed under the trade name Zavesca.
 | |
| Clinical data | |
|---|---|
| Trade names | Zavesca |
| Other names | 1,5-(butylimino)-1,5-dideoxy-D-glucitol, N-butyl-deoxynojirimycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Protein binding | Nil |
| Metabolism | Nil |
| Elimination half-life | 6–7 hours |
| Excretion | Renal, unchanged |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.074 |
| Chemical and physical data | |
| Formula | C10H21NO4 |
| Molar mass | 219.28 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Medical uses
Miglustat is used to treat adults with mild-to-moderate type I Gaucher disease for whom enzyme replacement therapy is unsuitable.[1] It was approved in Europe in 2002[2] and by the US FDA in 2003.[3]
Miglustat is the first treatment to be approved for treating progressive neurological complications in people with Niemann–Pick disease, type C (NPC); it has been approved in Europe in 2009, Canada in 2010, and Japan in 2012, but not in the US where the FDA declined to approve it in 2010 and called for more data.[4][5][6][7]
Contraindications
Miglustat is contraindicated for people with neurological conditions, kidney problems, women who are pregnant, and men and women planning to conceive a child.[8]
Adverse effects
Serious side effects include pain, burning, numbness or tingling in the hands, arms, legs, or feet; shaking hands that cannot be controlled; changes in vision; and easy bruising or bleeding. Common side effects include gastrointestinal effects (including diarrhea, stomach pain or bloating, gas, loss of appetite, weight loss, upset stomach, vomiting, constipation), dry mouth, muscular effects (including weakness, muscle cramps, especially in the legs, feeling of heaviness in the arms or legs, unsteadiness when walking), back pain, dizziness, nervousness, headache, memory problems, and difficult or irregular menstruation (period).[8]
Mechanism of action
Type I Gaucher's disease is an autosomal recessive disorder; parents are generally healthy carriers with one functional and one mutated (nonfunctioning) copy of the Gaucher disease gene, GBA. People with type I Gaucher have a defect in the enzyme called glucocerebrosidase (also known as acid β-glucosidase). Glucocerebrosidase is an enzyme, and its function is to convert glucocerebroside (also known as glucosylceramide) into ceramide and glucose. When this enzyme doesn't work, glucocerebroside accumulates, which in turn causes liver and spleen enlargement, changes in the bone marrow and blood, and bone disease.[9]
Other treatments on the market (imiglucerase (approved in 1995),[10] velaglucerase (approved in 2010),[11] taliglucerase alfa (Elelyso) (approved in 2012)[12]) are enzyme replacement therapy - they are functioning versions of the enzyme that doesn't work. Miglustat works differently - it prevents the formation of the substance that builds up when the enzyme doesn't work; this is called substrate reduction therapy.[13]
Chemistry
Miglustat is an iminosugar, a synthetic analogue of D-glucose[14] and a white to off-white crystalline solid that has a bitter taste.[15]
Research
In July 2004 Actelion started a clinical trial of miglustat to treat Tay–Sachs disease, particularly late-onset Tay–Sachs with an estimated enrollment of 10 subjects; the trial ended August 2007.[16]
In November 2007, Actelion initiated a clinical trial with miglustat in people with cystic fibrosis (CF) who have the ΔF508 in both copies of the cystic fibrosis transmembrane conductance regulator (CFTR) gene; the study ended in March 2008.[17] The cystic fibrosis trial showed no effect.[18]
See also
- Migalastat, a drug for the treatment of Fabry disease, with a similar structure
- Miglitol, an oral antidiabetic drug with a similar structure
References
- Cox, TM; et al. (2003). ": Advisory Council to the European Working Group on Gaucher Disease. The role of the iminosugar N-butyldeoxynojirimycin (miglustat) in the management of type I (non-neuronopathic) Gaucher disease: a position statement". J Inherit Metab Dis. 26 (6): 513–26. PMID 14605497.
- European Medicines Agency. Human Medicines Database. Zavesca (miglustat) Page Accessed 1 September 2014.
- Actelion Press Release August 2003. Zavesca approved -- first oral treatment option for type 1 Gaucher disease
- UK Medicines Information. New Drugs Online Report for miglustat
- Staff, The Pharma Letter. 4 April 2012. Actelion drops setipiprant, gets miglustat approval in Japan
- Kevin Grogan for PharmaTimes. 10 March 2010. FDA rejects Actelion's Zavesca for rare NP-C disease Archived 2014-09-03 at the Wayback Machine
- Actelion Press Release. 23 March 2010 Zavesca® (Miglustat) First Treatment Available in Canada for Rare Progressive Niemann-Pick Type C Disease
- American Society of Health-System Pharmacists, Inc. for the Public Library of Medicine. Miglustat on MedlinePlus Accessed 1 September 2014
- Grabowski, GA (2012). "Gaucher disease and other storage disorders". Hematology Am Soc Hematol Educ Program. 2012: 13–8. doi:10.1182/asheducation-2012.1.13. PMID 23233555.
- Deegan, PB; Cox, TM (2012). "Imiglucerase in the treatment of Gaucher disease: a history and perspective". Drug Des Devel Ther. 6: 81–106. doi:10.2147/DDDT.S14395. PMC 3340106. PMID 22563238.
- "Shire Announces FDA Approval Of VPRIV(TM) (velaglucerase Alfa For Injection) For The Treatment Of Type I Gaucher Disease". Medicalnewstoday.com. Retrieved 2012-08-13.
- Yukhananov, Anna (1 May 2012). "U.S. FDA approves Pfizer/Protalix drug for Gaucher". Chicago Tribune. Reuters. Retrieved 2 May 2012.
- Actelion. FDA Advisory Briefing Book for Miglustat (Ogt 918, Zavesca®) in Niemann-Pick Type C Disease NDA 021-348/S-007 Prepared for the Endocrinologic and Metabolic Drugs Advisory Committee meeting, 1 December 2009
- Abian O, Alfonso P, Velazquez-Campoy A, Giraldo P, Pocovi M, Sancho J (December 2011). "Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase". Molecular Pharmaceutics. 8 (6): 2390–7. doi:10.1021/mp200313e. PMID 21988669.
- European Medicines Agency 1 April 2003 Scientific discussion related to approval of Zavesca.
- Clinicaltrials.gov Pharmacokinetics, Safety and Tolerability of Zavesca (Miglustat) in Patients With Infantile Onset Gangliosidosis: Single and Steady State Oral Doses Accessed 1 September 2014
- Clinicaltrials.gov Miglustat / OGT 918 in the Treatment of Cystic Fibrosis Accessed 1 September 2014
- Leonard, A; et al. (May 2012). "A randomized placebo-controlled trial of miglustat in cystic fibrosis based on nasal potential difference". J Cyst Fibros. 11 (3): 231–6. doi:10.1016/j.jcf.2011.12.004. PMID 22281182.