Carglumic acid
Carglumic acid is an orphan drug, marketed by Orphan Europe under the trade name Carbaglu. Carglumic acid is used for the treatment of hyperammonaemia in patients with N-acetylglutamate synthase deficiency.[1][2] The initial daily dose ranges from 100 to 250 mg/kg, adjusted thereafter to maintain normal plasma levels of ammonia.
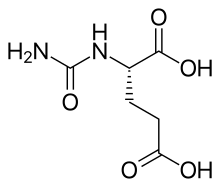 | |
| Clinical data | |
|---|---|
| Other names | (S)-2-ureidopentanedioic acid |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | Undetermined |
| Metabolism | Partial |
| Elimination half-life | 4.3 to 9.5 hours |
| Excretion | Fecal (60%) and kidney (9%, unchanged) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.116.323 |
| Chemical and physical data | |
| Formula | C6H10N2O5 |
| Molar mass | 190.2 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The US FDA approved it for treatment of hyperammonaemia on March 18, 2010. Orphan Drug exclusivity expired on March 18, 2017.[3]
Adverse effects
The most common adverse effects include vomiting, abdominal pain, fever, and tonsillitis.[4]
References
- Caldovic L, Morizono H, Daikhin Y, Nissim I, McCarter RJ, Yudkoff M, Tuchman M (2004). "Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate". J Pediatr. 145 (4): 552–4. doi:10.1016/j.jpeds.2004.06.047. PMID 15480384.
- Elpeleg O, Shaag A, Ben-Shalom E, Schmid T, Bachmann C (2002). "N-acetylglutamate synthase deficiency and the treatment of hyperammonemic encephalopathy". Ann Neurol. 52 (6): 845–9. doi:10.1002/ana.10406. PMID 12447942.
- "Patent and Exclusivity Search Results".
- Drugs.com: Professional Drug Facts for Carglumic Acid.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.