Uridine triacetate
Uridine triacetate (INN),[1] formerly known as vistonuridine, is an orally active tri-acetylated prodrug of uridine[2] used:
- in the treatment of hereditary orotic aciduria (brand name Xuriden /ˈzʊərədɛn/ ZOOR-ə-den);[3]
- to treat patients following an overdose of chemotherapy drugs 5-fluorouracil (5-FU) or capecitabine regardless of the presence of symptoms, or who exhibit early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration (brand name Vistogard).[4][5][6]
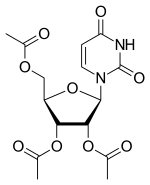 | |
| Clinical data | |
|---|---|
| Trade names | Vistogard, Xuriden |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616020 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral granules |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Pyrimidine catabolic pathway |
| Onset of action | Tmax = 2–3 hours |
| Elimination half-life | 2–2.5 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.021.710 |
| Chemical and physical data | |
| Formula | C15H18N2O9 |
| Molar mass | 370.314 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Uridine triacetate was developed, manufactured and distributed by Wellstat Therapeutics. It was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) and approved for use in the United States in 2015.[7][8][9]
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 65" (PDF). World Health Organization. p. 92. Retrieved 12 March 2017.
- "Uridine triacetate — DrugBank Page". 12 March 2017.
- "Xuriden (uridine triacetate) Oral Granules. Full Prescribing Information" (PDF). Wellstat Therapeutics Corporation. Gaithersburg, MD 20878. Retrieved 12 March 2017.
- "Vistogard (uridine triacetate) Oral Granules. Full Prescribing Information" (PDF). Wellstat Therapeutics Corporation. Gaithersburg, MD 20878. Retrieved 12 March 2017.
- "BTG Announces FDA Approval of Vistogard® (Uridine Triacetate) as Antidote to Overdose and Early Onset, Severe, or Life-Threatening Toxicities from Chemotherapy Drugs 5-Fluorouracil (5-FU) or Capecitabine". BTG International Ltd. 11 December 2015. Retrieved 12 March 2017.
- "Approved Drugs — Uridine Triacetate". U.S. Food and Drug Administration. Retrieved 12 March 2017.
- "Xuriden (uridine triacetate) oral granules". U.S. Food and Drug Administration (FDA). 8 October 2015. Archived from the original on 8 December 2019. Retrieved 7 December 2019.

- "Drug Trials Snapshots: Xuriden". U.S. Food and Drug Administration (FDA). 4 September 2015. Archived from the original on 8 December 2019. Retrieved 8 December 2019.

- "Previous Cumulative CY CDER BT Approvals". U.S. Food and Drug Administration (FDA). Archived from the original on 8 December 2019. Retrieved 7 December 2019.

External links
- "Uridine triacetate". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.