Management of prostate cancer
Treatment for prostate cancer may involve active surveillance, surgery, radiation therapy - including brachytherapy (prostate brachytherapy) and external-beam radiation therapy, proton therapy, high-intensity focused ultrasound (HIFU), cryosurgery, hormonal therapy, chemotherapy, or some combination. Treatments also extend to survivorship based interventions. These interventions are focused on five domains including: physical symptoms, psychological symptoms, surveillance, health promotion and care coordination.[1]. However, a published review has found only high levels of evidence for interventions that target physical and psychological symptom management and health promotion, with no reviews of interventions for either care coordination or surveillance.[2]. The favored treatment option depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors include the man's age, his general health, and his feelings about potential treatments and their possible side-effects. Because all treatments can have significant side-effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
| Management of prostate cancer | |
|---|---|
| Specialty | oncology |
The selection of treatment options may involve complex decisions with many factors. For example, radical prostatectomy after primary radiation failure, a very technically challenging surgical operation, may not be an option.[3] This may enter into the treatment decision.
If the cancer has spread beyond the prostate, treatment options change significantly, so most doctors who treat prostate cancer use a variety of nomograms to predict the probability of spread. Treatment by watchful waiting/active surveillance, HIFU, external-beam radiation therapy, brachytherapy, cryosurgery, and surgery are, in general, offered to men whose cancer remains within the prostate. Clinicians may reserve hormonal therapy and chemotherapy for disease that has spread beyond the prostate. However, there are exceptions: radiation therapy can treat some advanced tumors, and hormonal therapy some early-stage tumors. Doctors may also propose cryotherapy (the process of freezing the tumor), hormonal therapy, or chemotherapy if initial treatment fails and the cancer progresses.[4]
Active surveillance
Active surveillance is observation and regular monitoring without invasive treatment. In the context of prostate disease this usually comprises regular PSA blood tests and prostate biopsies. Active surveillance is often used when an early stage, slow-growing prostate cancer is suspected. However, watchful waiting may also be suggested when the risks of surgery, radiation therapy, or hormonal therapy outweigh the possible benefits. Other treatments can be started if symptoms develop, or if there are signs that the cancer growth is accelerating .
Approximately one-third of men who choose active surveillance for early stage tumors eventually have signs of tumor progression, and they may need to begin treatment within three years.[5] Men that choose active surveillance avoid the risks of surgery, radiation, and other treatments. The risk of disease progression and metastasis (spread of the cancer) may be increased, but this increase risk appears to be small if the program of surveillance is followed closely, generally including serial PSA assessments and repeat prostate biopsies every 1–2 years depending on the PSA trends.
Study results in 2011 suggest active surveillance is the best choice for older 'low-risk' patients.[6]
Surgery
Surgical removal of the prostate, or prostatectomy, is a common treatment either for early stage prostate cancer or for cancer that has failed to respond to radiation therapy. The most common type is radical retropubic prostatectomy, when the surgeon removes the prostate through an abdominal incision. Another type is radical perineal prostatectomy, when the surgeon removes the prostate through an incision in the perineum, the skin between the scrotum and anus. Radical prostatectomy can also be performed laparoscopically, through a series of small (1 cm) incisions in the abdomen, with or without the assistance of a surgical robot.
Radical prostatectomy
Radical prostatectomy is effective for tumors that have not spread beyond the prostate;[7] cure rates depend on risk factors such as PSA level and Gleason grade. However, it may cause nerve damage that may significantly alter the quality of life of the prostate cancer survivor. Radical prostatectomy has been associated with a greater decrease in sexual function and increased urinary incontinence than external beam radiotherapy, an alternative treatment.[8]
Radical prostatectomy has traditionally been used alone when the cancer is localized to the prostate. In the event of positive margins or locally advanced disease found on pathology, adjuvant radiation therapy may offer improved survival. Surgery may also be offered when a cancer is not responding to radiation therapy. However, because radiation therapy causes tissue changes, prostatectomy after radiation has higher risks of complications.
Laparoscopic radical prostatectomy, LRP, is a new way to approach the prostate surgically with intent to cure. Contrasted with the open surgical form of prostate cancer surgery, laparoscopic radical prostatectomy requires a smaller incision. Relying on modern technology, such as miniaturization, fiber optics, and the like, laparoscopic radical prostatectomy is a minimally invasive prostate cancer treatment but is technically demanding and seldom done in the USA.
- Robotic assistance
Some believe that in the hands of an experienced surgeon, robotic-assisted laparoscopic prostatectomy (RALP) may reduce positive surgical margins when compared to radical retropubic prostatectomy (RRP) among patients with prostate cancer according to a retrospective study.[9] The relative risk reduction was 57.7%. For patients at similar risk to those in this study (35.5% of patients had positive surgical margins following RRP), this leads to an absolute risk reduction of 20.5%. 4.9 patients must be treated for one to benefit (number needed to treat = 4.9). Other recent studies have shown RALP to result in a significantly higher rate of positive margins.[10] Other studies showed no difference of robotic to open surgery.[11] The relative merits of RALP and potential benefit versus open radical prostatectomy is currently an area of intense research and debate in urology. The only proven and accepted advantage to RALP is less intraoperative blood loss. Other suggested advantages beyond this lack definitive data and have not been widely accepted by the broader urological community.
Transurethral resection
Transurethral resection of the prostate, commonly called a "TURP," is a surgical procedure performed when the tube from the bladder to the penis (urethra) is blocked by prostate enlargement. In general, TURP is for benign disease and is not meant as definitive treatment for prostate cancer. During a TURP, a small instrument (cystoscope) is placed into the penis and the blocking prostate is cut away.
Cryosurgery
Cryosurgery is another method of treating prostate cancer in which the prostate gland is exposed to freezing temperatures.[12] Cryosurgery is less invasive than radical prostatectomy, and general anesthesia is less commonly used. Under ultrasound guidance, a method invented by Dr. Gary Onik,[13] metal rods are inserted through the skin of the perineum into the prostate. Highly purified argon gas is used to cool the rods, freezing the surrounding tissue at −186 °C (−302 °F). As the water within the prostate cells freezes, the cells die. The urethra is protected from freezing by a catheter filled with warm liquid. Impotence occurs up to ninety percent of the time.[14]
Surgical removal of the testicles
In metastatic disease, where cancer has spread beyond the prostate, removal of the testicles (called orchiectomy) may be done to decrease testosterone levels and control cancer growth. (See hormonal therapy, below).
Complications of surgery
The most common serious complications of surgery are loss of urinary control and impotence. Reported rates of both complications vary widely depending on how they are assessed, by whom, and how long after surgery, as well as the setting (e.g., academic series vs. community-based or population-based data). Although penile sensation and the ability to achieve orgasm usually remain intact, erection and ejaculation are often impaired. Medications such as sildenafil (Viagra), tadalafil (Cialis), or vardenafil (Levitra) may restore some degree of potency. For most men with organ-confined disease, a more limited "nerve-sparing" technique may help reduce urinary incontinence and impotence.[15]
Postoperative urinary incontinence has been reported at 16% among patients at 12 months following radical prostatectomy. Although pelvic floor muscle training has been prescribed to improve urinary continence, the evidence for efficacy in men after radical prostatectomy has come into question recently. According to information from the Men After Prostate Surgery (MAPS) randomised control trial, pelvic floor muscle training was not shown to be therapeutic or cost effective in improving urinary continence. Of the patients in the intervention group, 148 of the 196 patients reported some form of incontinence at the 12-month mark. In the control group, 151 of the 195 patients reported some urinary incontinence (EER=.755, CER=.774, RRR=.0245, ARR=.019, NNT=Not Significant).[16]
Radiation therapy
Radiation therapy, also known as radiotherapy, is often used to treat all stages of prostate cancer. It is also often used after surgery if the surgery was not successful at curing the cancer. Radiotherapy uses ionizing radiation to kill prostate cancer cells. When absorbed in tissue, ionizing radiation such as gamma and x-rays damage the DNA in cancer cells, which increases the probability of apoptosis (cell death). Normal cells are able to repair radiation damage, while cancer cells are not. Radiation therapy exploits this fact to treat cancer. Two different kinds of radiation therapy are used in prostate cancer treatment: external beam radiation therapy and brachytherapy (specifically prostate brachytherapy).
External beam radiation therapy
External beam radiation therapy (EBRT) uses a linear accelerator to produce high-energy x-rays that are directed in a beam towards the prostate. A technique called Intensity Modulated Radiation Therapy (IMRT) may be used to adjust the radiation beam to conform with the shape of the tumor, allowing higher doses to be given to the prostate and seminal vesicles with less damage to the bladder and rectum. External beam radiation therapy is generally given over several weeks, with daily visits to a radiation therapy center. New types of radiation therapy such as IMRT have fewer side effects than traditional treatment. However, in the short term, EBRT has been associated with acute worsening of urinary obstructive and bowel symptoms. These symptoms have been shown to decline over time.[8] Eleven centers in the United States are now using proton therapy for prostate cancer, which uses protons rather than X-rays to kill the cancer cells. Researchers are also studying types of stereotactic body radiotherapy (SBRT) to treat prostate cancer.[17]
Brachytherapy
Permanent implant brachytherapy is a popular treatment choice for patients with low to intermediate risk features, can be performed on an outpatient basis, and is associated with good 10-year outcomes with relatively low morbidity.[18] It involves the placement of about 100 small "seeds" containing radioactive material (such as iodine-125 or palladium-103) with a needle through the skin of the perineum directly into the tumor while under spinal or general anesthetic. These seeds emit lower-energy X-rays which are only able to travel a short distance. Although the seeds eventually become inert, they remain in the prostate permanently. The risk of exposure to others from men with implanted seeds is generally accepted to be insignificant.[19] However, men are encouraged to talk to their doctors about any special temporary precautions around small children and pregnant women.[20]
Uses
Radiation therapy is commonly used in prostate cancer treatment. It may be used instead of surgery or after surgery in early stage prostate cancer (adjuvant radiotherapy). Radiation treatments also can be combined with hormonal therapy for intermediate risk disease, when surgery or radiation therapy alone is less likely to cure the cancer. Some radiation oncologists combine external beam radiation and brachytherapy for intermediate to high-risk situations. Radiation therapy is often used in conjunction with hormone therapy for high-risk patients.[21] Others use a "triple modality" combination of external beam radiation therapy, brachytherapy, and hormonal therapy. In advanced stages of prostate cancer, radiation is used to treat painful bone metastases or reduce spinal cord compression.
Radiation therapy is also used after radical prostatectomy either for cancer recurrence or if multiple risk factors are found during surgery. Radiation therapy delivered immediately after surgery when risk factors are present (positive surgical margin, extracapsular extension, seminal vessicle involvement) has been demonstrated to reduce cancer recurrence, decrease distant metastasis, and increase overall survival in two separate randomized trials.[22]
Side effects
Side effects of radiation therapy might occur after a few weeks into treatment. Both types of radiation therapy may cause diarrhea and mild rectal bleeding due to radiation proctitis, as well as potential urinary incontinence and impotence. Symptoms tend to improve over time except erections which typically worsen as time progresses.
A new method to reduce rectal radiation injury in prostate cancer patients involves the use of an absorbable spacer placed between the prostate and rectum.
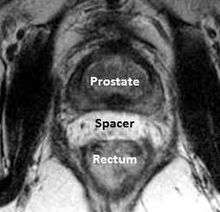
Such spacers are commercially available in some regions, and are undergoing clinical trials in others.[23] By temporarily altering the anatomy these products have the potential to allow for improved cancer targeting while minimizing risk to neighboring healthy tissues. Prostate Rectum Spacers should be compatible with all prostate cancer radiotherapy treatments including 3D conformal, IMRT and stereotactic radiation and brachytherapy.
Comparison to surgery
Multiple retrospective analyses have demonstrated that overall survival and disease-free survival outcomes are similar between radical prostatectomy, external beam radiation therapy, and brachytherapy.[24] However, a recent retrospective study suggests that men under 60 with high grade prostate cancer have higher survival rates with surgery than with beam radiation.[25] Rates for impotence when comparing radiation to nerve-sparing surgery are similar. Radiation has lower rates of incontinence compared with surgery, but has higher rates of occasional mild rectal bleeding.[26] Men who have undergone external beam radiation therapy may have a slightly higher risk of later developing colon cancer and bladder cancer.[27]
Since prostate cancer is generally a multifocal disease, the traditional prostatectomy eliminates all local lesions by removing the entire prostate. However, it has been hypothesized that an "index lesion" might be responsible for disease progression. Therefore, focal therapy targeted towards the index lesion might effectively treat prostate cancer while preserving the remainder of the gland. Interventional radiologists have started to treat prostate cancer with minimally invasive therapies such as cryoablation, HIFU, radiofrequency ablation, and photodynamic therapy that permit focal therapy by utilizing image guidance. These therapies are still in beginning or experimental stages; however, because they preserve tissue, they can potentially reduce adverse treatment outcomes such as impotence and incontinence. A small prospective study published in European Urology in February 2015 assessed the focal treatment of index lesions with HIFU in patients with multifocal prostate cancer and found that the majority of men returned to baseline genitourinary function and 86% of men were free of clinically significant prostate cancer at one year.[28] Small, nonrandomized cohort studies with a median range follow-up 17–47 months have shown that cryoablation, HIFU, and phototherapy are associated with low rates of adverse effects and early disease control rates of 83%-100% based on negative biopsies.[29]
Patients who might particularly benefit from focal therapy with HIFU are men with recurrent cancer after the gland has been removed. Cancer recurrence rates after surgical resection can be as high as 15-20%. MR imaging improves early detection of cancer, so MR-guided therapies can be applied to treat recurrent disease. Additionally, for men who have already failed salvage radiation treatment and have limited therapeutic options remaining, interventional therapies might offer more chances to potentially cure their disease. While recent studies have demonstrated the feasibility of these treatments, additional work is needed to further evaluate which patients are best suited for these procedures and determine long-term efficacy.[30]
High intensity focused ultrasound
High intensity focused ultrasound (HIFU) was first used in the 1940s and 1950s in efforts to destroy tumors in the central nervous system. Since then, HIFU has been shown to be effective at destroying malignant tissue in the brain, prostate, spleen, liver, kidney, breast, and bone.[31]
HIFU for prostate cancer utilizes ultrasound to ablate/destroy the tissue of the prostate. During the HIFU procedure, sound waves are used to heat the prostate tissue, thus destroying the cancerous cells. In essence, ultrasonic waves are focused on specific areas of the prostate to eliminate the prostate cancer, with minimal risks of affecting other tissue or organs. Temperatures at the focal point of the sound waves can exceed 100 °C (212 °F).[31] However, many studies of HIFU were performed by manufacturers of HIFU devices, or members of manufacturers' advisory panels.[32]
Contraindications to HIFU for prostate cancer include a prostate volume larger than 40 grams, which can prevent targeted HIFU waves from reaching the anterior and anterobasal regions of the prostate, anatomic or pathologic conditions that may interfere with the introduction or displacement of the HIFU probe into the rectum, and high-volume calcification within the prostate, which can lead to HIFU scattering and transmission impairment.[33]
A 2012 UK trial of focal HIFU on 41 patients reported no histological evidence of cancer in 77% of men treated (95% confidence interval: 61 - 89%) at 12 month targeted biopsy, and a low rate of genitourinary side effects.[34] However, this does not necessarily mean that 77% of men were definitively cured of prostate cancer, since systematic and random sampling errors are present in the biopsy process, and therefore recurrent or previously undetected cancer can be missed.[35]
Life-style changes
Prostate enlargement can cause difficulties emptying the bladder completely. This situation, in which there is residual volume in the bladder is prone to complications such as cystitis and bladder stones, also commonly found in patients with benign prostate hyperplasia. It was often suggested to change the voiding position of symptomatic males, however study results showed heterogeneity. A meta-analysis of people with prostate enlargement and healthy males showed a significant reduction of residual volume, while a trend towards an improved urinary flow rate and decreased voiding time was found.[36] The effect of changing ones position is thought to arise from relaxation of the pelvic musculature, which are contracted in the standing position thereby influencing urodynamics.
Frequent exercise such as brisk walking can delay the progress of prostate cancer[37][38]
Hormonal therapy
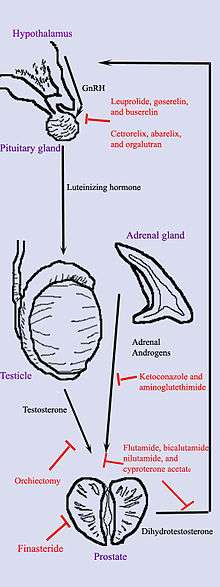
Androgen deprivation therapy
Hormonal therapy uses medications or surgery to block prostate cancer cells from getting dihydrotestosterone (DHT), a hormone produced in the prostate and required for the growth and spread of most prostate cancer cells. Blocking DHT often causes prostate cancer to stop growing and even shrink. However, hormonal therapy rarely cures prostate cancer because cancers that initially respond to hormonal therapy typically become resistant after one to two years. Hormonal therapy is, therefore, usually used when cancer has spread from the prostate. It may also be given to certain men undergoing radiation therapy or surgery to help prevent return of their cancer.[39]
Hormonal therapy for prostate cancer targets the pathways the body uses to produce DHT. A feedback loop involving the testicles, the hypothalamus, and the pituitary, adrenal, and prostate glands controls the blood levels of DHT. First, low blood levels of DHT stimulate the hypothalamus to produce gonadotropin-releasing hormone (GnRH). GnRH then stimulates the pituitary gland to produce luteinizing hormone (LH), and LH stimulates the testicles to produce testosterone. Finally, testosterone from the testicles and dehydroepiandrosterone from the adrenal glands stimulate the prostate to produce more DHT. Hormonal therapy can decrease levels of DHT by interrupting this pathway at any point. There are several forms of hormonal therapy:
- Orchiectomy, also called "castration," is surgery to remove the testicles. Because the testicles make most of the body's testosterone, after orchiectomy testosterone levels drop. Now the prostate not only lacks the testosterone stimulus to produce DHT but also does not have enough testosterone to transform into DHT. Orchiectomy is considered the gold standard of treatment.[40]
- Antiandrogens are medications such as flutamide, nilutamide, bicalutamide, enzalutamide, apalutamide, and cyproterone acetate that directly block the actions of testosterone and DHT within prostate cancer cells.
- Medications that block the production of adrenal androgens such as DHEA include ketoconazole and aminoglutethimide. Because the adrenal glands make only about 5% of the body's androgens, these medications are, in general, used only in combination with other methods that can block the 95% of androgens made by the testicles. These combined methods are called total androgen blockade (TAB). TAB can also be achieved using antiandrogens.
- GnRH action can be interrupted in one of two ways. GnRH antagonists such as abarelix and degarelix suppress the production of LH directly by acting on the anterior pituitary. GnRH agonists such as leuprorelin and goserelin suppress LH through the process of downregulation after an initial stimulation effect which can cause initial tumor flare. In order to prevent stimulation of tumor growth during the initial LH surge, an antiandrogen such as cyproterone acetate is prescribed a week before and three weeks after GnRH agonists are given. Abarelix and degarelix are examples of GnRH antagonists, whereas the GnRH agonists include leuprolide, goserelin, triptorelin, and buserelin. Initially, GnRH agonists increase the production of LH. However, because the constant supply of the medication does not match the body's natural production rhythm, production of both LH and GnRH decreases after a few weeks.[41]
- Abiraterone acetate was FDA approved in April 2011 for treatment of castration-resistant prostate cancer for patients who have failed docetaxel therapy. Abiraterone acetate inhibits an enzyme known as CYP17, which is used in the body to produce testosterone.[42][43]
The most successful hormonal treatments are orchiectomy and GnRH agonists. Despite their higher cost, GnRH agonists are often chosen over orchiectomy for cosmetic and emotional reasons. Eventually, total androgen blockade may prove to be better than orchiectomy or GnRH agonists used alone.
Each treatment has disadvantages that limit its use in certain circumstances. Although orchiectomy is a low-risk surgery, the psychological impact of removing the testicles can be significant, and sterility is certain. The loss of testosterone can cause hot flashes, weight gain, loss of libido, enlargement of the breasts (gynecomastia), impotence, penile atrophy, and osteoporosis. GnRH agonists eventually cause the same side effects as orchiectomy but may cause worse symptoms at the beginning of treatment. When GnRH agonists are first used, testosterone surges can lead to increased bone pain from metastatic cancer, so antiandrogens or abarelix is often added to blunt these side effects. Estrogens are not commonly used because they increase the risk for cardiovascular disease and blood clots. In general, the antiandrogens do not cause impotence, and usually cause less loss of bone and muscle mass. Ketoconazole can cause liver damage with prolonged use, and aminoglutethimide can cause skin rashes.
Estrogen therapy
High-dose estrogen therapy is used in the treatment of prostate cancer.[44] Estrogens that have been used include diethylstilbestrol, fosfestrol, ethinylestradiol, ethinylestradiol sulfonate, polyestradiol phosphate, and estradiol undecylate, as well as the dual estrogenic and cytostatic agent estramustine phosphate.[44][45] Newer estrogens with improved tolerability and safety like GTx-758 have also been studied.[46][47] Estrogens are effective in prostate cancer because they are functional antiandrogens.[45][48] They both suppress testosterone levels into the castrate range via their antigonadotropic effects[45][48] and they reduce the fraction of free and bioavailable testosterone by increasing sex hormone-binding globulin levels.[46][48] Estrogens may also have direct cytotoxic effects in the prostate gland.[45]
Estrogens have been found to be equivalent in effectiveness to androgen deprivation therapy via surgical or medical castration and nonsteroidal antiandrogens.[48] In addition, they prevent hot flashes, preserve bone density, preserve some sexual interest, have quality-of-life advantages, and are far less costly than conventional androgen deprivation therapy.[49][50][51][52][53][54][55] However, estrogens cause feminization and gynecomastia as side effects.[50][51][52][54][55] Moreover, at a dosage of 3 to 5 mg/day, diethylstilbestrol can increase cardiovascular mortality – particularly in those patients who already have a compromised cardiovascular system. Diethylstilbestrol at 1 to 2 mg/day appears to be safe and effective for CRPC patients who have healthy cardiovascular systems and who concurrently take low-dose aspirin.[48] Although the most commonly employed estrogens, oral and synthetic estrogens such as diethylstilbestrol and ethinylestradiol, increase cardiovascular mortality, certain estrogens, namely bioidentical parenteral estrogens such as polyestradiol phosphate and high-dose transdermal estradiol, do not do so; this is attributed to different degrees of effect of the estrogen classes on liver protein synthesis and by extension coagulation factors.[48]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Recurrent disease
After surgery or radiation, PSA may start to rise again, which is called biochemical recurrence if a certain threshold is met in PSA levels (typically 0.1 or 0.2 ng/ml). After surgery, salvage radiation therapy, often in combination with hormonal therapy, may be offered.
Extensive disease
Palliative care for advanced stage prostate cancer focuses on extending life and relieving the symptoms of metastatic disease. As noted above, abiraterone is showing some promise in treating advance-stage prostate cancer. It causes a dramatic reduction in PSA levels and tumor sizes in aggressive advanced-stage prostate cancer for 70% of patients.[42][43] Chemotherapy may be offered to slow disease progression and postpone symptoms. The most commonly used regimen combines the chemotherapeutic drug docetaxel with a corticosteroid such as prednisone. One study showed that treatment with docetaxel with prednisone prolonged life from 16.5 months for those taking mitoxantrone and prednisone to 18.9 months for those taking docetaxel + prednisone.[56] Bisphosphonates such as zoledronic acid have been shown to delay skeletal complications such as fractures or the need for radiation therapy in patients with hormone-refractory metastatic prostate cancer.[57] Xofigo is a new alpha emitting pharmaceutical targeting bone metastasis. The phase II testing shows prolonged patient survival times, reduced pain, and improved quality of life.
Bone pain due to metastatic disease is treated with opioid pain relievers such as morphine and oxycodone. External beam radiation therapy directed at bone metastases may provide pain relief. Injections of certain radioisotopes, such as strontium-89, phosphorus-32, or samarium-153, also target bone metastases and may help relieve pain.
Alternative therapies
As an alternative to active surveillance or definitive treatments, other therapies are also under investigation for the management of prostate cancer. PSA has been shown to be lowered in men with apparent localized prostate cancer using a vegan diet (fish allowed), regular exercise, and stress reduction.[58] These results have so far proven durable after two-years' treatment. However, this study did not compare the vegan diet to either active surveillance or definitive treatment, and thus cannot comment on the comparative efficacy of the vegan diet in treating prostate cancer.[59]
Many other single agents have been shown to reduce PSA, slow PSA doubling times, or have similar effects on secondary markers in men with localized cancer in short term trials, such as pomegranate juice or genistein, an isoflavone found in various legumes.[60][61]
The potential of using multiple such agents in concert, let alone combining them with lifestyle changes, has not yet been studied. A more thorough review of natural approaches to prostate cancer has been published.[62]
Neutrons have been shown to be superior to X-rays in the treatment of prostatic cancer. The rationale is that tumours containing hypoxic cells (cells with enough oxygen concentration to be viable, yet not enough to be X-ray-radiosensitive) and cells deficient in oxygen are resistant to killing by X-rays. Thus, the lower Oxygen Enhancement Ratio (OER) of neutrons confers an advantage. Also, neutrons have a higher relative biological effectiveness (RBE) for slow-growing tumours than X-rays, allowing for an advantage in tumour cell killing.[63]
Prevention
Neither selenium nor vitamin E have been found to be effective in preventing prostate cancer.[64]
Trade-offs
The trade-off dilemma refers to the choice between the expected beneficial and harmful effects in terms of survival and quality of life for a particular treatment. An example of such trade-off in prostate cancer treatment includes urinary and bowel symptoms and waning sexual function.[65] How common these symptoms are and the distress they cause varies between types of treatment and individuals.[66]
One option is to trade off an intact sexual function for the possibility of a prolonged life expectancy by not having curative treatment. The choice involves a trade-off so it is of central importance for the person and the physician to have access to information on established treatment benefits and side effects. A Swedish study found that the willingness to do this kind of trade-off varied considerably among men.[65] While six out of ten were willing to consider a trade-off between life expectancy and intact sexual function, given the present knowledge of treatment benefits for clinically localized prostate cancer, four out of ten stated that they would under all circumstances choose treatment irrespective of the risk for waning sexual function. Access to valid empirical information is crucial for such decision making. Key factors here are an individual’s feeling towards the illness, their emotional values and religious beliefs. A substantial proportion of people and physicians, experience stress in judging the trade-off between different treatment options and treatment side-effects which adds to the stress of cancer diagnosed, a situation made worse in that eight out of ten people with prostate cancer have no one to confide in except their spouse and one out of five live in total emotional isolation.[67]
See also
References
- Resnick, Matthew J.; Lacchetti, Christina; Bergman, Jonathan; Hauke, Ralph J.; Hoffman, Karen E.; Kungel, Terrence M.; Morgans, Alicia K.; Penson, David F. (2015). "Prostate Cancer Survivorship Care Guideline: American Society of Clinical Oncology Clinical Practice Guideline Endorsement". Journal of Clinical Oncology. 33 (9): 1078–1085. doi:10.1200/JCO.2014.60.2557. PMID 25667275.
- Crawford-Williams, Fiona; March, Sonja; Goodwin, Belinda C.; Ralph, Nicholas; Galvão, Daniel A.; Newton, Robert U.; Chambers, Suzanne K.; Dunn, Jeff (2018). "Interventions for prostate cancer survivorship: A systematic review of reviews" (PDF). Psycho-Oncology. 27 (10): 2339–2348. doi:10.1002/pon.4888. PMID 30255558.
- Mouraviev V, Evans B, Polascik TJ (2006). "Salvage prostate cryoablation after primary interstitial brachytherapy failure: a feasible approach". Prostate Cancer and Prostatic Diseases. 9 (1): 99–101. doi:10.1038/sj.pcan.4500853. PMID 16314889.
- "Prostate Cancer At A Glance". shavemagazine.com.
- Wu, H; Sun L; Moul JW; Wu HY; McLeod DG; Amling C; Lance R; Kusuda L; Donahue T; Foley J; Chung A; Sexton W; Soderdahl D (March 2004). "Watchful waiting and factors predictive of secondary treatment of localized prostate cancer". Journal of Urology. 171 (3): 1111–6. doi:10.1097/01.ju.0000113300.74132.8b. PMID 14767282.
- http://www.cancer.gov/ncicancerbulletin/041911/page2 Active Surveillance May Be Preferred Option in Some Men with Prostate Cancer Archived May 3, 2011, at the Wayback Machine
- Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, Spångberg A, Busch C, Nordling S, Garmo H, Palmgren J, Adami HO, Norlén BJ, Johansson JE (May 2005). "Radical prostatectomy versus watchful waiting in early prostate cancer". New England Journal of Medicine. 352 (19): 1977–84. doi:10.1056/NEJMoa043739. PMID 15888698.
- Chen C, Chen Z, Wang K, Hu L, Xu R, He X (November 2017). "Comparisons of health-related quality of life among surgery and radiotherapy for localized prostate cancer: a systematic review and meta-analysis". Oncotarget. 8 (58): 99057–99065. doi:10.18632/oncotarget.21519. PMC 5716791. PMID 29228751.
- Smith JA, Chan RC, Chang SS, et al. (December 2007). "A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy". Journal of Urology. 178 (6): 2385–9, discussion 2389–90. doi:10.1016/j.juro.2007.08.008. PMID 17936849.
- Ou, YC; Yang CR; Wang J; Cheng CL; Patel VR (May 2009). "Comparison of Robotic-assisted versus Retropubic Radical Prostatectomy Performed by a Single Surgeon". Anticancer Research. 29 (5): 1637–42. PMID 19443379.
- Ham, WS; Park SY; Rha KH; Kim WT; Choi YD (June 2009). "Robotic radical prostatectomy for patients with locally advanced prostate cancer is feasible: results of a single-institution study". Journal of Laparoendoscopic & Advanced Surgical Techniques. 19 (3): 329–32. doi:10.1089/lap.2008.0344. PMID 19397390.
- PreventProstateCancer.info: A Brief Overview of Prostate Cancer Archived 2008-09-24 at the Wayback Machine
- "Cryosurgical system for destroying tumors by freezing". 1994-08-02. Archived from the original on 2009-03-29.
- Bahn, DK; Lee F; Badalament R; Kumar A; Greski J; Chernick M (August 2002). "Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer". Urology. 60 (2 Suppl 1): 3–11. doi:10.1016/S0090-4295(02)01678-3. PMID 12206842.
- Gerber GS, Thisted RA, Scardino PT, Frohmuller HG, Schroeder FH, Paulson DF, Middleton AW Jr, Rukstalis DB, Smith JA Jr, Schellhammer PF, Ohori M, Chodak GW (August 28, 1996). "Results of radical prostatectomy in men with clinically localized prostate cancer". JAMA: The Journal of the American Medical Association. 276 (8): 615–9. doi:10.1001/jama.276.8.615. PMID 8773633.
- Glazener C, Boachie C, Buckley B, et al. (July 2011). "Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials". The Lancet. 378 (9788): 328–37. doi:10.1016/S0140-6736(11)60751-4. hdl:2164/2366. PMID 21741700.
- "Archived copy". Archived from the original on 2009-07-15. Retrieved 2009-08-06.CS1 maint: archived copy as title (link)
- Nag S, Beyer D, Friedland J, Grimm P, Nath R (July 1999). "American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer". International Journal of Radiation Oncology, Biology, Physics. 44 (4): 789–99. doi:10.1016/S0360-3016(99)00069-3. PMID 10386635.
- Perez, CA; Hanks GE; Leibel SA; Zietman AL; Fuks Z; Lee WR (December 1, 1993). "Localized carcinoma of the prostate (stages T1B, T1C, T2, and T3). Review of management with external beam radiation therapy". Cancer. 72 (11): 3156–73. doi:10.1002/1097-0142(19931201)72:11<3156::AID-CNCR2820721106>3.0.CO;2-G. PMID 7694785. Review.
- http://www.rtanswers.com/treatmentinformation/cancertypes/prostate/prostatebrach.aspx
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW (2004). "6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial". JAMA. 292 (7): 821–7. doi:10.1001/jama.292.7.821. PMID 15315996.
- Thompson IM, Tangen CM, Paradelo J (2009). "Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial". Journal of Urology. 181 (3): 956–62. doi:10.1016/j.juro.2008.11.032. PMC 3510761. PMID 19167731.
- "Products — Augmenix". Augmenix.com. Retrieved 2012-02-16.
- Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA (August 2002). "Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy". Journal of Clinical Oncology. 20 (16): 3376–85. doi:10.1200/JCO.2002.01.150. PMID 12177097.
- Huang H, Muscatelli S, Naslund M, Badiyan SN, Kaiser A, Siddiqui MM (Jan 2019). "Evaluation of Cancer Specific Mortality with Surgery versus Radiation as Primary Therapy for Localized High Grade Prostate Cancer in Men Younger Than 60 Years". Journal of Urology. 201: 120–128. doi:10.1016/j.juro.2018.07.049.
- Lawton, CA; Won M; Pilepich MV; Asbell SO; Shipley WU; Hanks GE; Cox JD; Perez CA; Sause WT; Doggett SR; et al. (September 1991). "Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706". International Journal of Radiation Oncology, Biology, Physics. 21 (4): 935–9. doi:10.1016/0360-3016(91)90732-J. PMID 1917622.
- Brenner, DJ; Curtis RE; Hall EJ; Ron E (January 15, 2000). "Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery". Cancer. 88 (2): 398–406. CiteSeerX 10.1.1.385.7956. doi:10.1002/(SICI)1097-0142(20000115)88:2<398::AID-CNCR22>3.0.CO;2-V. PMID 10640974.
- Ahmed, Hashim U.; Dickinson, Louise; Charman, Susan; Weir, Shraddha; McCartan, Neil; Hindley, Richard G.; Freeman, Alex; Kirkham, Alex P.; Sahu, Mahua; Scott, Rebecca; Allen, Clare; Van Der Meulen, Jan; Emberton, Mark (2015). "Focal Ablation Targeted to the Index Lesion in Multifocal Localised Prostate Cancer: A Prospective Development Study". European Urology. 68 (6): 927–936. doi:10.1016/j.eururo.2015.01.030. PMID 25682339.
- Karavitakis, Markos; Ahmed, Hashim U.; Abel, Paul D.; Hazell, Steven; Winkler, Mathias H. (2011). "Tumor focality in prostate cancer: Implications for focal therapy". Nature Reviews Clinical Oncology. 8 (1): 48–55. doi:10.1038/nrclinonc.2010.190. PMID 21116296.
- Society of Interventional Radiology. "The Hot - And Cold - Interventional Radiology Treatments For Recurrent Prostate Cancer". www.biocompare.com. Biocompare: The buyers guide for life scientists. Retrieved 18 April 2018.
- Gardner TA, Koch MO (December 2005). "Prostate cancer therapy with high-intensity focused ultrasound". Clinical Genitourinary Cancer. 4 (3): 187–92. doi:10.3816/CGC.2005.n.031. PMID 16425987.
- Pickles, Tom; Goldenberg, Larry; Steinhoff, Gary (2005). "High-Intensity Focused Ultrasound for Prostate Cancer" (PDF). British Columbia Cancer Agency.
- Barqawi AB, Crawford ED (2008). "Emerging Role of HIFU as a Noninvasive Ablative Method to Treat Localized Prostate Cancer". Oncology. 22 (2): 123–9, discussion 129, 133, 137 passim. PMID 18409659.
- Ahmed, Hashim U; Hindley, Richard G; Dickinson, Louise; Freeman, Alex; Kirkham, Alex P; Sahu, Mahua; Scott, Rebecca; Allen, Clare; Van der Meulen, Jan; Emberton, Mark (1 June 2012). "Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study". The Lancet Oncology. 13 (6): 622–632. doi:10.1016/S1470-2045(12)70121-3. PMC 3366323. PMID 22512844.
- Ahmed, Hashim Uddin; Moore, Caroline; Lecornet, Emilie; Emberton, Mark (1 May 2010). "Focal Therapy in Prostate Cancer: Determinants of Success and Failure". Journal of Endourology. 24 (5): 819–825. doi:10.1089/end.2009.0665. PMID 20380513.
- de Jong Y, Pinckaers JH, ten Brinck RM, Lycklama à Nijeholt AA, Dekkers OM (2014). "Urinating standing versus sitting: position is of influence in men with prostate enlargement. A systematic review and meta-analysis". PLOS ONE. 9 (7): e101320. Bibcode:2014PLoSO...9j1320D. doi:10.1371/journal.pone.0101320. PMC 4106761. PMID 25051345.
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM (February 2011). "Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study". Journal of Clinical Oncology. 29 (6): 726–32. doi:10.1200/JCO.2010.31.5226. PMC 3056656. PMID 21205749.
- Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM (June 2011). "Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor". Cancer Research. 71 (11): 3889–95. doi:10.1158/0008-5472.CAN-10-3932. PMC 3107352. PMID 21610110.
- Robson, M; Dawson N (June 1996). "How is androgen-dependent metastatic prostate cancer best treated?". Hematology/Oncology Clinics of North America. 10 (3): 727–47. doi:10.1016/S0889-8588(05)70364-6. PMID 8773508. Review.
- "Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group". British Journal of Urology. 79 (2): 235–46. February 1997. doi:10.1046/j.1464-410x.1997.d01-6840.x. PMID 9052476.
- Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben-Josef E, Middleton R, Porterfield H, Sharp SA, Smith TJ, Taplin ME, Vogelzang NJ, Wade JL Jr, Bennett CL, Scher HI, American Society of Clinical Oncology (July 15, 2004). "American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer". Journal of Clinical Oncology. 22 (14): 2927–41. doi:10.1200/JCO.2004.04.579. PMID 15184404. (Erratum: doi:10.1200/JCO.2004.08.943)
- de Bono, Johann; Gerhardt Attard; Alison H.M. Reid; Timothy A. Yap; Florence Raynaud; Mitch Dowsett; Sarah Settatree; Mary Barrett; Christopher Parker; Vanessa Martins; Elizabeth Folkerd; Jeremy Clark; Colin S. Cooper; Stan B. Kaye; David Dearnaley; Gloria Lee (July 21, 2004). "Phase I Clinical Trial of a Selective Inhibitor of CYP17, Abiraterone Acetate, Confirms That Castration-Resistant Prostate Cancer Commonly Remains Hormone Driven". Journal of Clinical Oncology. 26 (14): 4563–4571. doi:10.1200/JCO.2007.15.9749. PMID 18645193. (Erratum: doi:10.1200/JCO.2012.43.7756)
- Richard Warry (July 22, 2008). "Drug for deadly prostate cancer". BBC. Retrieved 2008-07-23.
- Christoffel Jos van Boxtel; Budiono Santoso; I. Ralph Edwards (2008). Drug Benefits and Risks: International Textbook of Clinical Pharmacology. IOS Press. pp. 458–. ISBN 978-1-58603-880-9.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 540–542. doi:10.1007/978-3-642-60107-1. ISBN 978-3-642-60107-1.
- Coss CC, Jones A, Parke DN, Narayanan R, Barrett CM, Kearbey JD, Veverka KA, Miller DD, Morton RA, Steiner MS, Dalton JT (March 2012). "Preclinical characterization of a novel diphenyl benzamide selective ERα agonist for hormone therapy in prostate cancer". Endocrinology. 153 (3): 1070–81. doi:10.1210/en.2011-1608. PMID 22294742.
- Yu EY, Getzenberg RH, Coss CC, Gittelman MM, Keane T, Tutrone R, et al. (February 2015). "Selective estrogen receptor alpha agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer". European Urology. 67 (2): 334–41. doi:10.1016/j.eururo.2014.06.011. PMID 24968970.
- Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- Ali Shah SI (2015). "Emerging potential of parenteral estrogen as androgen deprivation therapy for prostate cancer". South Asian Journal of Cancer. 4 (2): 95–7. doi:10.4103/2278-330X.155699. PMC 4418092. PMID 25992351.
- Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocrine-Related Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- Wibowo E, Wassersug RJ (September 2013). "The effect of estrogen on the sexual interest of castrated males: Implications to prostate cancer patients on androgen-deprivation therapy". Critical Reviews in Oncology/Hematology. 87 (3): 224–38. doi:10.1016/j.critrevonc.2013.01.006. PMID 23484454.
- Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". Journal of Urology. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (February 2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". British Journal of Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clinical Genitourinary Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nature Clinical Practice. Oncology. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators (October 7, 2004). "Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer". New England Journal of Medicine. 351 (15): 1502–12. doi:10.1056/NEJMoa040720. PMID 15470213.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002). "A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma". Journal of the National Cancer Institute. 94 (19): 1458–68. doi:10.1093/jnci/94.19.1458. PMID 12359855.
- Ornish, D; Weidner G; Fair WR; et al. (2005). "Intensive lifestyle changes may affect the progression of prostate cancer". Journal of Urology. 174 (3): 1065–70. doi:10.1097/01.ju.0000169487.49018.73. PMID 16094059.
- Frattaroli J, Weidner G, Dnistrian AM, et al. (December 2008). "Clinical events in prostate cancer lifestyle trial: results from two years of follow-up". Urology. 72 (6): 1319–23. doi:10.1016/j.urology.2008.04.050. PMID 18602144.
- Pantuck, AJ; Leppert JT; Zomorodian N; et al. (2006). "Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer". Clinical Cancer Research. 12 (13): 4018–26. doi:10.1158/1078-0432.CCR-05-2290. PMID 16818701.
- Kumar, NB; Cantor A; Allen K; et al. (2004). "The specific role of isoflavones in reducing prostate cancer risk". The Prostate. 59 (2): 141–7. doi:10.1002/pros.10362. PMID 15042614.
- Yarnell, Eric (1999). "A Naturopathic Approach to Prostate Cancer Part 2: Guidelines for Treatment and Prevention". Alternative and Complementary Therapies. 5 (6): 360–368. doi:10.1089/act.1999.5.360.
- Hall, Eric J. (2000). Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Williams. pp. 432–3. ISBN 978-0-06-141077-2.
- Lippman SM, Klein EA, Goodman PJ, et al. (January 2009). "Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT)". JAMA. 301 (1): 39–51. doi:10.1001/jama.2008.864. PMC 3682779. PMID 19066370.
- Helgason ÁR, Adolfsson J, Dickman P, Fredrikson M, Arver S, Steineck G (1996). "Waning sexual function - the most important disease-specific distress for patients with prostate cancer". British Journal of Cancer. 73 (11): 1417–1421. doi:10.1038/bjc.1996.268. PMC 2074472. PMID 8645589.
- Helgason ÁR, Adolfsson J, Dickman P, Fredrikson M, Steineck G (1998). "Distress due to unwanted side-effects of prostate cancer treatment is related to impaired well-being (quality of life)". Prostate Cancer and Prostatic Diseases. 1 (3): 128–133. doi:10.1038/sj.pcan.4500226. PMID 12496905.
- Helgason ÁR, Dickman PW, Adolfsson J, Steineck G (2001). "Emotional isolation : Prevalence and the effect on well-being among 50-80 year old prostate cancer patients". Scandinavian Journal of Urology and Nephrology. 35 (2): 97–101. CiteSeerX 10.1.1.549.5736. doi:10.1080/003655901750170407. PMID 11411666.