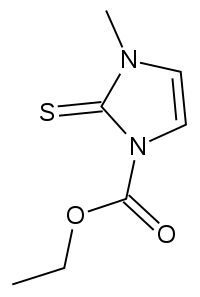
Carbimazole
Carbimazole is used to treat hyperthyroidism. Carbimazole is a pro-drug as after absorption it is converted to the active form, methimazole. Methimazole prevents thyroid peroxidase enzyme from coupling and iodinating the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 (thyroxine).
 | |
| Clinical data | |
|---|---|
| Trade names | Neo-mercazole |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 85% |
| Elimination half-life | 5.3h |
| Excretion | >90%Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.762 |
| Chemical and physical data | |
| Formula | C7H10N2O2S |
| Molar mass | 186.233 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Medical uses
Medical therapy for hyperthyroidism typically involves either titrating the dose of Carbimazole until the patient becomes euthyroid or maintaining a high dose of Carbimazole to suppress endogenous thyroid production, and then replacing thyroid hormone with levothyroxine ("block and replace"). Treatment is usually given for 18–24 months followed by a trial withdraw.[1]
The onset of anti-thyroid effect is rapid but the onset of clinical effects on thyroid hormone levels in the blood is much slower. This is because the large store of pre-formed T3 and T4 in the thyroid gland and bound to thyroid binding globulin (99% bound) has to be depleted before any beneficial clinical effect occurs.
Adverse effects
Whilst rashes and pruritus are common, these can often be treated with antihistamines without stopping the carbimazole. For those patients where sensitivity reactions can not be controlled, propylthiouracil may be used as an alternative; cross-sensitivity between these drugs is rare.
Its most serious rare side effect is bone marrow suppression causing neutropenia and agranulocytosis. This may occur at any stage during treatment and without warning; monitoring of white cell count is not useful. Patients are advised to immediately report symptoms of infection, such as sore throat or fever, so that a full blood count test may be arranged. If this confirms a low neutrophil count, discontinuation of the drug leads to recovery. However failure to report suggestive symptoms or delays in considering the possibility of immunosuppression and its testing, can lead to fatalities.
Precautions
Some people are allergic to azole(s). Some azole drugs have adverse side-effects. Some azole drugs may disrupt estrogen production in pregnancy, affecting pregnancy outcome.[2]
Carbimazole should be used judiciously in pregnancy as it crosses the placenta. It has (rarely) been associated with congenital defects, including aplasia cutis of the neonate but is not contra-indicated. However, it more predictably may cause fetal hypothyroidism so (in minimal doses) it can be used in order to control maternal hyperthyroidism. There are reported cases of goiter and choanal atresia in fetus.[3]</ref> Furthermore, breast feeding is possible but only if lowest effective dose is used and neonatal development is closely monitored.
For the above reasons, it is preferable to use PTU in pregnancy, especially in the first trimester, with the possibility of changing to Carbimazole for the second and third trimesters.[4]
Brand names
- Neo-mercazole[5]
- Vidalta
- Thyrocab
- Neomerdin
See also
References
- Lawrence N, Cheetham T, Elder C (September 2019). "How do paediatricians use and monitor antithyroid drugs in the UK? A clinician survey". Clinical Endocrinology. 3 (91): 417–423. doi:10.1111/cen.14046. PMID 31179554.
- Kragie L, Turner SD, Patten CJ, Crespi CL, Stresser DM (August 2002). "Assessing pregnancy risks of azole antifungals using a high throughput aromatase inhibition assay". Endocrine Research. 28 (3): 129–40. doi:10.1081/ERC-120015045. PMID 12489563.
- Brunton L, Chabner BA, Knollman B (2011). Goodman & Gilman's pharmacological basis of therapeutics (12th ed.). McGraw-Hill. ISBN 978-0-07-162442-8.
- Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN (July 2009). "The Role of Propylthiouracil in the Management of Graves' Disease in Adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration". Thyroid. 19 (7): 673–4. doi:10.1089/thy.2009.0169. PMID 19583480.
- "Neo-Mercazole Carbimazole". Nicholas Laboratories Indonesia.
Further reading
- British National Formulary. 45. March 2003. ISBN 978-0-85369-555-4.