Thiamazole
Thiamazole, also known as methimazole, is a medication used to treat hyperthyroidism.[2] This includes Graves disease, toxic multinodular goiter, and thyrotoxic crisis.[2] It is taken by mouth.[2] Full effects may take a few weeks to occur.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tapazole, others |
| Other names | thiamazole (INN, BAN); methimazole (USAN); MMI |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682464 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | None |
| Metabolism | Liver |
| Elimination half-life | 5-6 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.439 |
| Chemical and physical data | |
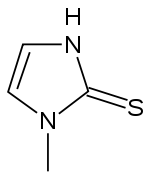
| Formula | C4H6N2S |
| Molar mass | 114.17 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 146 °C (295 °F) |
| Solubility in water | 275[1] mg/mL (20 °C) |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include itchiness, hair loss, nausea, muscle pain, swelling, and abdominal pain.[2] Severe side effects may include low blood cell counts, liver failure, and vasculitis.[2] Use is not recommended during the first trimester of pregnancy but may be used in the second trimester or third trimester.[4] It may be used during breastfeeding.[4] Those who developed significant side effects may also have problems with propylthiouracil.[2] Thiamazole is a thioamide and works by decreasing the production of thyroid hormones.[2]
Thiamazole was approved for medical use in the United States in 1950.[2] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[5] It is available as a generic medication.[2] A month supply in the United States has a wholesale cost of about US$6.80.[6] It is also available in Europe and Asia.[7] In 2016, it was the 275th most prescribed medication in the United States with more than a million prescriptions.[8]
Medical uses
Thiamazole is a drug used to treat hyperthyroidism such as in Graves' disease, a condition that occurs when the thyroid gland begins to produce an excess of thyroid hormone. The drug may also be taken before thyroid surgery to lower thyroid hormone levels and minimize the effects of thyroid manipulation. Additionally, thiamazole is used in the veterinary setting to treat hyperthyroidism in cats.
Adverse effects
It is important to monitor any symptoms of fever or sore throat while taking thiamazole; this could indicate the development of agranulocytosis, an uncommon but severe side effect resulting from a drop in the white blood cell count (to be specific, neutropenia, a deficiency of neutrophils). A complete blood count (CBC) with differential is performed to confirm the suspicion, in which case the drug is discontinued.[9] Administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) may increase recovery.
Other known side effects include:
- skin rash
- itching
- abnormal hair loss
- upset stomach
- vomiting
- loss of taste
- abnormal sensations (tingling, prickling, burning, tightness, and pulling)
- swelling
- joint and muscle pain
- drowsiness
- dizziness
- decreased platelet count (thrombocytopenia)
- aplasia cutis congenita (prenatal exposure)
- thyroid gland enlargement (prenatal exposure)
- choanal atresia (prenatal exposure during the first trimester of pregnancy)
Interactions
Adverse effects may occur for individuals who:
- Take anticoagulants ('blood thinners') such as warfarin (Coumadin), diabetes medications, digoxin (Lanoxin), theophylline (Theobid, Theo-Dur), and vitamins
- Have ever had any blood disease, such as decreased white blood cells (leukopenia), decreased platelets (thrombocytopenia) or aplastic anemia, or liver disease (hepatitis, jaundice)
Mechanism of action
Thiamazole inhibits the enzyme thyroperoxidase, which normally acts in thyroid hormone synthesis by oxidizing the anion iodide (I−) to iodine (I2), hypoiodous acid (HOI), enzyme linked hypoiodate (EOI) facilitating iodine's addition to tyrosine residues on the hormone precursor thyroglobulin, a necessary step in the synthesis of triiodothyronine (T3) and thyroxine (T4).
It does not inhibit the action of the sodium-dependent iodide transporter located on follicular cells' basolateral membranes. Inhibition of this step requires competitive inhibitors such as perchlorate and thiocyanate.
It acts at CXCL10.[10]
Chemical properties
The imidazole derivative thiamazole is a white to matte brown crystalline powder with a characteristic odour. The boiling point is 280 °C (decomposition). Thiamazole is soluble in water, ethanol and chloroform, but hardly soluble in ether.[11] Galenic preparations are injectable solutions and tablets.
Thiamazole acts as a free radical scavenger for radicals such as the hydroxyl radical (•OH) radical.[12] It is used as free radical scavenger in organic chemistry.[13]
References
- "DrugBank: Methimazole (DB00763)". drugbank.ca. Retrieved 21 July 2015.
- "Methimazole Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 8 April 2019.
- Spina, Domenico (2008). The Flesh and Bones of Medical Pharmacology E-Book. Elsevier Health Sciences. p. 74. ISBN 9780723437161.
- "Methimazole Use During Pregnancy". Drugs.com. Retrieved 8 April 2019.
- "World Health Organization model list of essential medicines: 21st list 2019". 2019. hdl:10665/325771. Cite journal requires
|journal=(help) - "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- Jastrzębska, Helena (2015). "Antithyroid drugs". Thyroid Research. 8 (Suppl 1): A12. doi:10.1186/1756-6614-8-S1-A12. PMC 4480840.
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- Fumarola, A; Di Fiore, A; Dainelli, M; Grani, G; Calvanese, A (November 2010). "Medical treatment of hyperthyroidism: state of the art". Experimental and Clinical Endocrinology & Diabetes. 118 (10): 678–84. doi:10.1055/s-0030-1253420. PMID 20496313.
- Crescioli C, Cosmi L, Borgogni E, et al. (October 2007). "Methimazole inhibits CXC chemokine ligand 10 secretion in human thyrocytes". J. Endocrinol. 195 (1): 145–55. doi:10.1677/JOE-07-0240. PMID 17911406.
- Entry on Thiamazol. at: Römpp Online. Georg Thieme Verlag, retrieved 10. November 2014.
- Taylor, J.J.; Willson, R.L.; Kendall-Taylor, P. (29 October 1984). "Evidence for direct interactions between methimazole and free radicals". FEBS Letters. 176 (2): 337–340. doi:10.1016/0014-5793(84)81192-8.
- Inhibition of amine oxide, 30 December 2010, retrieved 14 April 2019